What Is Valence Sodium? A Simple Explanation
Valence sodium, a term that might seem obscure to some, is actually a fascinating concept that bridges the realms of chemistry and materials science. To break it down simply, valence sodium refers to the electronic state of sodium when it’s part of a compound, particularly in its +1 oxidation state. This means that a sodium atom loses one electron to form a positively charged ion, known as a cation. The term “valence” itself refers to the combining capacity of an element, essentially how many bonds it can form with other elements.
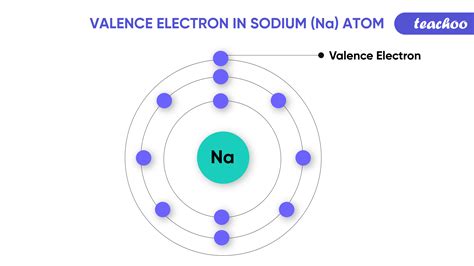
In the case of sodium, its valence electron is the outermost electron in its atomic structure. Sodium has one valence electron in its outer shell, which it readily loses to achieve a stable electronic configuration. This process of losing or gaining electrons to form ions is fundamental in the formation of many compounds and is a key aspect of chemistry. For instance, when sodium reacts with chlorine (a highly electronegative element), it loses its valence electron to chlorine, resulting in the formation of sodium chloride, or common table salt, where sodium is in its +1 valence state.
The unique behavior of sodium in its compounds is what makes valence sodium an interesting topic of study. Sodium’s ability to easily lose its electron makes it highly reactive, especially with nonmetals. This reactivity is utilized in various applications, from the manufacturing of soap and paper to the production of certain dyes and pharmaceuticals. Moreover, the study of valence sodium has implications in understanding the properties of materials at a fundamental level, including their electrical conductivity, thermal properties, and reactivity.
One of the areas where understanding valence sodium is crucial is in the development of batteries. Sodium, being abundant and having a similar chemical behavior to lithium (a key component in many battery types), is being explored as a potential material for sodium-ion batteries. These batteries could offer a more sustainable and cost-effective alternative to lithium-ion batteries, which are currently dominant in the market. The valence state of sodium in these batteries dictates its ability to insert into and extract from the electrode materials, making the understanding of its valence state critical for optimizing battery performance.
Furthermore, the concept of valence sodium extends beyond the realm of inorganic chemistry into biology. Sodium ions play a crucial role in many biological processes, including nerve signal transmission and muscle contraction. The movement of sodium ions across cell membranes is essential for generating the electrical impulses that enable these functions. Understanding the valence state of sodium in biological systems can provide insights into how these processes occur and how they might be affected by changes in sodium concentration or by diseases that alter sodium handling in the body.
In conclusion, valence sodium is more than just a chemical concept; it underpins a wide range of chemical, materials, and biological phenomena. Its simplicity belies its importance, from the everyday compounds we use, like table salt, to the complex biological processes that keep us alive. As research continues to delve into the properties and applications of sodium and its compounds, the understanding of valence sodium will remain a foundational aspect of advancing our knowledge in these fields.
For those looking to delve deeper into the subject, consider exploring the following areas: - Sodium-ion Batteries: Research the latest developments in sodium-ion battery technology, including the materials being explored for the electrodes and the electrolytes, and how these batteries could disrupt the energy storage market. - Biological Roles of Sodium: Investigate the biological processes that rely on sodium, including action potentials in neurons and muscle contraction mechanisms. Understanding these can provide insights into diseases related to sodium imbalance. - Chemical Reactivity of Sodium: Study the chemical properties of sodium and its compounds, including its reactions with different elements and how these reactions are utilized in industrial processes.
Exploring Valence Sodium Further
- Start with the basics: Understand the atomic structure of sodium and how it loses its valence electron to form a positive ion.
- Dive into applications: Look into how sodium's reactivity is used in manufacturing and potential future applications like sodium-ion batteries.
- Explore biological aspects: Learn about the role of sodium in biological systems, including nerve impulse transmission and muscle function.
- Stay updated: Follow the latest research in fields related to sodium, from materials science to biology, to see how our understanding of valence sodium evolves.
In the realm of science, concepts like valence sodium serve as a reminder of the intricate connections between different fields of study and the potential for discovery that lies at these intersections. Whether you’re a seasoned chemist, a curious student, or simply someone interested in how the world works, the concept of valence sodium offers a fascinating glimpse into the underlying principles that govern our universe.
What is the valence electron of sodium, and why is it significant?
+The valence electron of sodium is the single electron in its outer shell. It's significant because sodium readily loses this electron to form a positive ion, which is crucial for its chemical reactivity and biological functions.
How does the concept of valence sodium apply to battery technology?
+In the context of battery technology, understanding the valence state of sodium is essential for developing sodium-ion batteries. The ability of sodium to insert into and extract from electrode materials depends on its valence state, affecting the battery's performance and efficiency.
What are some common compounds of sodium, and how are they used?
+Common compounds of sodium include sodium chloride (table salt), sodium hydroxide (used in manufacturing soap and paper), and sodium carbonate (used in glass production). These compounds exploit the reactivity of sodium with other elements to form useful materials.


