Sodium Lewis Dot Made Easy
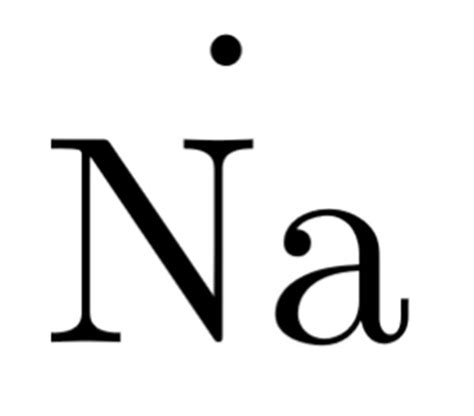
Understanding the Lewis dot structure for sodium, or any element, is fundamental in chemistry as it helps in visualizing how electrons are arranged around the nucleus of an atom. Sodium, with the atomic number 11, is in the first column of the periodic table, known as the alkali metals. Its electron configuration is 1s² 2s² 2p⁶ 3s¹, which means it has one valence electron.
To draw the Lewis dot structure for sodium, follow these simple steps:
Write the Symbol: Start by writing the symbol for sodium, which is Na.
Determine the Valence Electrons: Sodium has one valence electron, as indicated by its electron configuration ending in 3s¹.
Draw the Dots: Since sodium has one valence electron, you will draw one dot around the symbol for sodium. By convention, we usually start by placing this dot on the right side of the symbol and then move clockwise, but for a single electron, it can be placed on any side.
The resulting Lewis dot structure for sodium looks like this: Na· (please note that due to limitations, the actual dot may not be visible, but it’s represented as a dot next to the Na).
Understanding Reactivity: The single electron in the outermost shell of sodium makes it highly reactive. Sodium tends to lose one electron to achieve a noble gas configuration (that of neon), thus forming a +1 ion. This reactivity is characteristic of all alkali metals.
Formation of Compounds: When sodium reacts with nonmetals, such as chlorine (Cl₂), it loses its single valence electron to form a positively charged sodium ion (Na⁺) and two negatively charged chloride ions (Cl⁻), resulting in the formation of sodium chloride (NaCl), or common table salt.
Advantages of Lewis Dot Structures
- Visual Learning: Lewis structures provide a visual representation of the electrons in an atom or molecule, making it easier to understand chemical bonding and reactivity.
- Predicting Reactivity: By looking at a Lewis dot structure, you can predict the reactivity of an atom or molecule based on the number of valence electrons and whether they are paired or unpaired.
- Understanding Chemical Bonds: Lewis structures help in understanding how chemical bonds are formed, which is crucial for predicting the properties and behaviors of compounds.
Common Mistakes and Considerations
- Incorrect Counting of Electrons: Always ensure that you accurately count the number of valence electrons for the atom you are drawing the Lewis structure for.
- Not Following Octet Rule: Except for hydrogen, which can form only one bond (due to having only one orbital), most other atoms aim to achieve a full outer shell with eight electrons (the octet rule).
- Forgetting to Consider Formal Charges: In molecules, especially those with multiple bonds or lone pairs, considering formal charges can help in determining the most stable Lewis structure.
In conclusion, the Lewis dot structure for sodium is straightforward, reflecting its simple electron configuration and reactivity pattern. Understanding Lewis structures is a fundamental step in mastering chemistry, as it provides insights into the electronic structure of atoms and molecules, thereby explaining their chemical properties and reactivity.
Future Trends in Chemical Education
As technology and educational methods evolve, the way we teach and learn about Lewis structures and chemistry, in general, is also changing. Interactive tools and software are being developed to help students visualize and manipulate molecular structures in 3D, enhancing their understanding of chemical bonding and reactions.
Practical Applications of Sodium
Sodium and its compounds have numerous practical applications across various industries:
- Sodium in Food: Sodium chloride (NaCl) is a crucial component of our diet, used as a seasoning and in food preservation.
- Manufacturing Processes: Sodium is used in the production of paper, textiles, and gasoline.
- Pharmaceuticals: Sodium compounds are used in the manufacture of certain drugs.
These applications underscore the significant role sodium plays in everyday life, from the food we eat to the industrial processes that produce the goods we use.
Historical Evolution of Understanding Sodium
The discovery and understanding of sodium have evolved over centuries, with significant contributions from various scientists:
- Early Alchemists: Recognized the substance but did not fully understand its properties.
- Sir Humphry Davy: Successfully isolated sodium in 1807 through electrolysis, paving the way for modern chemistry.
This historical context provides a fascinating backdrop to the current understanding of sodium and its place in the periodic table, highlighting the progress made in chemistry and the foundational role of early scientists.
Decision Framework for Learning Chemistry
For students and enthusiasts looking to deepen their understanding of chemistry, including topics like Lewis structures, consider the following decision framework:
- Start with the Basics: Ensure a solid grasp of atomic structure, electron configuration, and the periodic table.
- Practice with Examples: Use online tools and textbooks to practice drawing Lewis structures for various atoms and molecules.
- Apply to Real-World Scenarios: Look for examples of how chemical principles are applied in industries, medicine, and everyday life.
- Stay Updated: Follow scientific journals and educational resources to stay current with the latest discoveries and teaching methods in chemistry.
By following this framework, learners can build a comprehensive understanding of chemistry, starting from foundational concepts like Lewis structures and moving on to more complex topics and applications.
FAQ Section
What is the electron configuration of sodium?
+The electron configuration of sodium is 1s² 2s² 2p⁶ 3s¹.
Why is sodium highly reactive?
+Sodium is highly reactive because it has one valence electron, which it readily loses to achieve a noble gas configuration, thus forming a positive ion.
What are some common uses of sodium?
+Sodium and its compounds are used in food (as sodium chloride), in manufacturing processes, and in the production of certain drugs.
Understanding sodium and its properties through the lens of Lewis dot structures not only enriches one’s knowledge of chemistry but also reveals the intricate and fascinating world of atoms and molecules that underpin our daily lives. As we continue to explore and apply chemical principles, we open doors to new discoveries and innovations that can transform industries and societies.


