Sodium Hydroxide Hydrochloric Acid
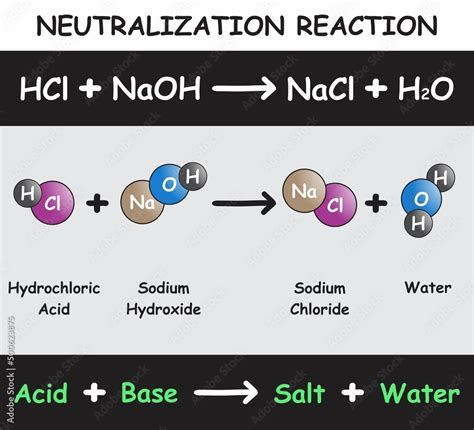
The reaction between sodium hydroxide (NaOH) and hydrochloric acid (HCl) is a fundamental concept in chemistry, representing a neutralization reaction where an acid and a base react to form salt and water. This reaction is not only crucial for understanding basic chemical principles but also has widespread applications in various industries, including manufacturing, pharmaceuticals, and environmental treatment.
Chemical Reaction
The chemical equation for the reaction between sodium hydroxide and hydrochloric acid is as follows:
NaOH (aq) + HCl (aq) → NaCl (aq) + H₂O (l)
In this equation, sodium hydroxide (NaOH), a strong base, reacts with hydrochloric acid (HCl), a strong acid, to produce sodium chloride (NaCl), which is common table salt, and water (H₂O). Both the reactants and the products are aqueous (dissolved in water), except for water, which is in its liquid state.
Process of Neutralization
Neutralization is a chemical reaction in which an acid and a base react to form a salt and water. In the case of sodium hydroxide and hydrochloric acid, the hydroxide ion (OH-) from the base combines with the hydrogen ion (H+) from the acid to form water, while the sodium ion (Na+) from the base combines with the chloride ion (Cl-) from the acid to form sodium chloride.
This reaction is exothermic, meaning it releases heat. The extent of heat released can be significant if the reaction is carried out with concentrated solutions, emphasizing the need for careful handling and appropriate safety measures.
Applications
The reaction between sodium hydroxide and hydrochloric acid has numerous practical applications: - Manufacturing: Sodium chloride (the product of this reaction) is used in the production of paper, dyes, and textiles. Additionally, the reaction itself is utilized in the manufacture of soaps, where fats are treated with sodium hydroxide to produce the fatty acid salts that constitute soap. - Pharmaceuticals: The control of pH levels is critical in pharmaceutical manufacturing. Sodium hydroxide and hydrochloric acid are often used to adjust the pH of solutions during the synthesis of drugs. - Environmental Treatment: In wastewater treatment, sodium hydroxide can be used to neutralize acidic wastewater, while hydrochloric acid can be used to neutralize basic wastewater, bringing the pH to a level suitable for discharge or further treatment. - Laboratory Settings: This reaction is frequently used in educational settings to demonstrate chemical reactions and the principles of acid-base chemistry.
Safety Considerations
Both sodium hydroxide and hydrochloric acid are hazardous substances that require careful handling: - Sodium Hydroxide: Highly caustic and can cause severe burns upon contact with skin or eyes. It is essential to wear protective gear, including gloves, goggles, and a face mask, when handling NaOH. - Hydrochloric Acid: Highly corrosive and toxic. Inhalation of its fumes can cause respiratory issues, and skin contact can lead to burns. Similar protective measures as for sodium hydroxide should be taken.
Conclusion
The reaction between sodium hydroxide and hydrochloric acid is a classic example of an acid-base neutralization reaction. Understanding this reaction is vital not only for academic purposes but also for applying its principles in various industrial and environmental applications. By recognizing the chemical processes involved and taking necessary safety precautions, individuals can harness the benefits of this reaction while minimizing its risks.
FAQ Section
What is the primary product of the reaction between sodium hydroxide and hydrochloric acid?
+The primary products are sodium chloride (common salt) and water.
Is the reaction between sodium hydroxide and hydrochloric acid exothermic or endothermic?
+This reaction is exothermic, releasing heat.
What safety precautions should be taken when handling sodium hydroxide and hydrochloric acid?
+Protective gear such as gloves, goggles, and a face mask should be worn. It’s also important to work in a well-ventilated area and follow proper disposal procedures.