So4 2 Conjugate Acid
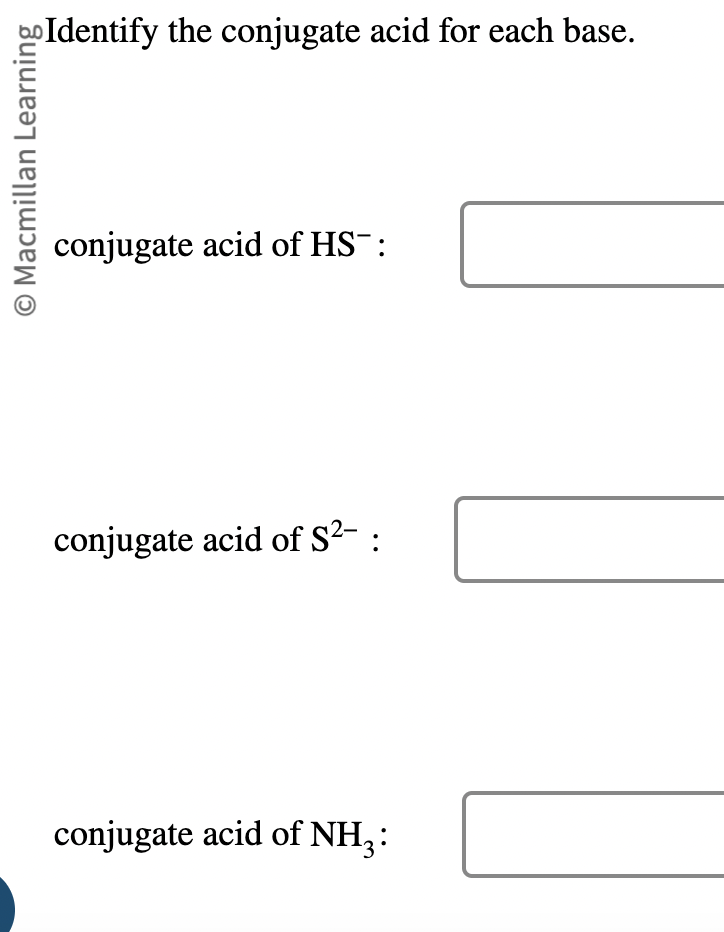
The sulfate ion, SO4 2-, is a conjugate base, and its conjugate acid is HSO4 - (hydrogen sulfate ion). To understand this relationship, it’s essential to delve into the concepts of acid-base chemistry, specifically the Bronsted-Lowry theory, which defines acids as proton donors and bases as proton acceptors.
In the context of the sulfate ion, when it acts as a base, it accepts a proton (H+), forming its conjugate acid, HSO4 -. This process can be represented by the following equation:
SO4 2- + H+ ⇌ HSO4 -
The hydrogen sulfate ion (HSO4 -) can further donate a proton, acting as an acid, to form the sulfate ion (SO4 2-) once again, and water (H2O). This is illustrated by the equation:
HSO4 - + H2O ⇌ SO4 2- + H3O+
This interconversion between SO4 2- and HSO4 - highlights the dynamic nature of acid-base reactions in aqueous solutions. The sulfate ion’s ability to accept and donate protons, albeit indirectly through its conjugate acid, showcases its role in maintaining the acid-base balance in various chemical and biological systems.
Understanding Conjugate Acid-Base Pairs
Conjugate acid-base pairs are crucial in understanding how acids and bases interact in chemical reactions. A conjugate acid is the species that results when a base accepts a proton, while a conjugate base is the species left after an acid donates a proton. The relationship between an acid and its conjugate base, or a base and its conjugate acid, is fundamental to acid-base chemistry.
For the sulfate ion (SO4 2-), its conjugate acid, HSO4 -, plays a significant role in many applications, including environmental science, where the sulfur cycle is vital, and in industrial processes, where sulfate and hydrogen sulfate ions are used in the manufacture of various products.
Acid Dissociation Constants
The strength of an acid is often quantified by its acid dissociation constant (Ka), which measures the extent to which an acid dissociates in water to produce hydrogen ions (H+) and its conjugate base. For HSO4 -, the first dissociation step (Ka1) involves the reaction:
HSO4 - + H2O ⇌ SO4 2- + H3O+
The Ka value for this reaction is around 1.02 x 10^-2 at 25°C, indicating that HSO4 - is a weak acid. This weakness as an acid reflects the stability of the sulfate ion and the energy required for the protonation and deprotonation reactions.
Biological and Environmental Relevance
Sulfate ions and their conjugate acids play significant roles in biological systems. Sulfate is an essential nutrient for plants, necessary for the synthesis of certain amino acids, among other compounds. In humans, sulfate ions are involved in various metabolic processes, including the synthesis and maintenance of connective tissue.
In environmental science, sulfate ions are a component of acid rain, which is formed when sulfur dioxide (SO2), a pollutant from the burning of fossil fuels, reacts with water and oxygen in the atmosphere. The resulting sulfuric acid (H2SO4) can dissociate into hydrogen ions and sulfate ions, contributing to the acidity of rain and affecting aquatic ecosystems.
Conclusion
The relationship between the sulfate ion (SO4 2-) and its conjugate acid, hydrogen sulfate ion (HSO4 -), is a critical aspect of acid-base chemistry. Understanding this relationship provides insights into the behavior of these species in various chemical and biological processes. The dynamic interplay between acids and their conjugate bases is fundamental to many natural and industrial processes, highlighting the importance of continued study and appreciation of acid-base principles.
What is the conjugate acid of the sulfate ion?
+The conjugate acid of the sulfate ion (SO4 2-) is the hydrogen sulfate ion (HSO4 -).
What is the significance of conjugate acid-base pairs in chemistry?
+Conjugate acid-base pairs are essential in understanding acid-base reactions, as they describe the relationship between an acid and its conjugate base or a base and its conjugate acid, which is fundamental to many chemical processes.
How is the strength of an acid quantified?
+The strength of an acid is typically quantified by its acid dissociation constant (Ka), which measures the extent to which an acid dissociates in water to produce hydrogen ions and its conjugate base.