Reducing A Ketone

Ketones are a type of organic compound that contains a carbonyl group, which is a carbon atom double-bonded to an oxygen atom. Reducing a ketone involves converting it into a different compound by adding hydrogen atoms to the carbonyl group, resulting in the formation of a new bond. This process is known as reduction, and it is a fundamental reaction in organic chemistry.
There are several ways to reduce a ketone, including the use of reducing agents such as lithium aluminum hydride (LiAlH4), sodium borohydride (NaBH4), and hydrogen gas (H2) in the presence of a catalyst. The choice of reducing agent depends on the specific ketone being reduced, as well as the desired product.
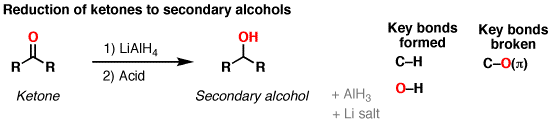
One of the most common methods for reducing a ketone is the use of lithium aluminum hydride (LiAlH4). This reducing agent is highly reactive and can reduce a wide range of ketones to their corresponding alcohols. The reaction involves the addition of LiAlH4 to the ketone, followed by the addition of water to quench the reaction and produce the alcohol.
Another method for reducing a ketone is the use of sodium borohydride (NaBH4). This reducing agent is less reactive than LiAlH4, but it is still highly effective at reducing ketones to their corresponding alcohols. The reaction involves the addition of NaBH4 to the ketone, followed by the addition of acid to quench the reaction and produce the alcohol.
Hydrogen gas (H2) can also be used to reduce a ketone, in the presence of a catalyst such as palladium or platinum. This method is known as catalytic hydrogenation, and it involves the addition of hydrogen gas to the ketone, followed by the absorption of the hydrogen atoms onto the catalyst. The resulting hydrogenated compound is then released from the catalyst, producing the reduced ketone.
The reduction of a ketone can result in the formation of a variety of compounds, including alcohols, alkanes, and alkyl halides. The specific product formed depends on the reducing agent used, as well as the reaction conditions.
It's worth noting that the reduction of a ketone can be a complex process, and the outcome can depend on a variety of factors, including the specific ketone being reduced, the reducing agent used, and the reaction conditions. Therefore, it's essential to carefully consider these factors when planning a reduction reaction.
Mechanism of Ketone Reduction
The mechanism of ketone reduction involves the addition of a reducing agent to the carbonyl group of the ketone, resulting in the formation of a new bond. The specific mechanism depends on the reducing agent used, but it generally involves a series of steps, including:
- Nucleophilic attack: The reducing agent attacks the carbonyl group of the ketone, resulting in the formation of a tetrahedral intermediate.
- Proton transfer: A proton is transferred to the oxygen atom of the carbonyl group, resulting in the formation of an alkoxide ion.
- Reduction: The alkoxide ion is reduced by the reducing agent, resulting in the formation of an alcohol.
Step-by-Step Mechanism of Ketone Reduction
- Nucleophilic attack: The reducing agent attacks the carbonyl group of the ketone.
- Proton transfer: A proton is transferred to the oxygen atom of the carbonyl group.
- Reduction: The alkoxide ion is reduced by the reducing agent.
Applications of Ketone Reduction
The reduction of ketones has a wide range of applications in organic chemistry, including:
- Synthesis of alcohols: Ketone reduction is a common method for synthesizing alcohols, which are used in a variety of applications, including the production of pharmaceuticals and agrochemicals.
- Synthesis of alkyl halides: Ketone reduction can also be used to synthesize alkyl halides, which are used in a variety of applications, including the production of pharmaceuticals and materials.
- Production of fragrances: Ketone reduction is used in the production of fragrances, such as vanillin, which is a common flavoring agent.
Advantages and Disadvantages of Ketone Reduction
| Advantages | Disadvantages |
|---|---|
| Highly effective method for reducing ketones | Can be sensitive to reaction conditions |
| Wide range of applications | Can result in the formation of side products |
Conclusion
In conclusion, the reduction of a ketone is a fundamental reaction in organic chemistry, and it has a wide range of applications in the synthesis of alcohols, alkyl halides, and other compounds. The choice of reducing agent depends on the specific ketone being reduced, as well as the desired product. By understanding the mechanism of ketone reduction and the factors that affect the reaction, chemists can design and optimize reduction reactions to produce the desired products.
What is the most common method for reducing a ketone?
+The most common method for reducing a ketone is the use of lithium aluminum hydride (LiAlH4) or sodium borohydride (NaBH4).
What are the advantages of ketone reduction?
+The advantages of ketone reduction include its high effectiveness, wide range of applications, and the ability to produce a variety of compounds, including alcohols and alkyl halides.
What are the disadvantages of ketone reduction?
+The disadvantages of ketone reduction include its sensitivity to reaction conditions and the potential for the formation of side products.
In the context of organic chemistry, the reduction of a ketone is a vital reaction that has been extensively studied and applied in various fields. By understanding the principles and mechanisms of ketone reduction, researchers and scientists can develop new methods and applications for this reaction, leading to the creation of new compounds and materials with unique properties.

