Polarity Of Hcl
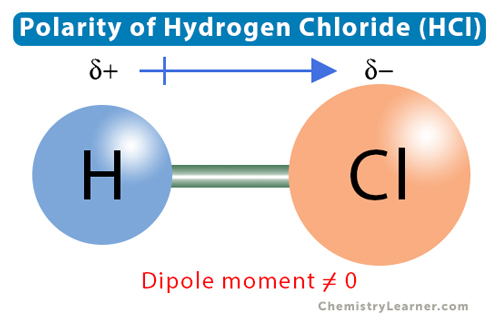
Understanding the polarity of HCl, or hydrochloric acid, is fundamental in chemistry due to its widespread presence in various chemical and biological processes. HCl is a diatomic molecule composed of hydrogen (H) and chlorine (Cl), with the hydrogen atom bonded to the chlorine atom through a covalent bond. This bond is polar due to the significant difference in electronegativity between hydrogen and chlorine.
Electronegativity Difference
Electronegativity is a measure of an atom’s ability to attract and hold onto electrons in a covalent bond. Chlorine has a higher electronegativity compared to hydrogen. On the Pauling scale, which is commonly used to measure electronegativity, chlorine has an electronegativity value of approximately 3.16, while hydrogen’s value is about 2.20. This difference in electronegativity (roughly 0.96 units) leads to an unequal sharing of the electrons in the covalent bond between hydrogen and chlorine, resulting in a polar bond.
Polarity of the HCl Molecule
The polarity of the HCl molecule arises from the unequal sharing of electrons, leading to a partial positive charge (δ+) on the hydrogen atom and a partial negative charge (δ-) on the chlorine atom. This creates a dipole moment, which is a measure of the separation of positive and negative electrical charges within the molecule. The dipole moment of HCl is approximately 1.05 debye (D), indicating a significant separation of charge and thus polarity.
Implications of Polarity
The polarity of HCl has several implications for its chemical behavior: - Solubility in Water: HCl is highly soluble in water because the polar water molecules can form hydrogen bonds with the partial negative charge on the chlorine atom of HCl, facilitating its dissolution. - Reactivity: The polarity of HCl influences its reactivity, particularly in its ability to donate a proton (H+), making it a strong acid. - Physical Properties: Polarity affects the physical properties of HCl, such as its boiling point. Polar molecules tend to have higher boiling points due to the stronger intermolecular forces (dipole-dipole interactions) between molecules.
Intermolecular Forces
In addition to the polarity within a single HCl molecule, the intermolecular forces between HCl molecules also play a crucial role in determining its physical properties. The primary intermolecular forces acting between HCl molecules are dipole-dipole interactions, where the partial positive charge on one molecule is attracted to the partial negative charge on another. These interactions are stronger than London dispersion forces (present in non-polar molecules) but generally weaker than hydrogen bonding (as seen in water or ammonia).
Conclusion
In conclusion, the polarity of HCl is a critical aspect of its chemical nature, arising from the difference in electronegativity between hydrogen and chlorine. This polarity influences various properties of HCl, including its solubility, reactivity, and physical characteristics. Understanding the polarity of molecules like HCl is essential for predicting their behavior in different chemical environments and for applying this knowledge in various fields, from chemistry and biology to engineering and pharmaceutical sciences.
What is the primary reason for the polarity in the HCl molecule?
+The primary reason for the polarity in the HCl molecule is the significant difference in electronegativity between the hydrogen and chlorine atoms, leading to an unequal sharing of electrons in the covalent bond.
How does the polarity of HCl affect its solubility in water?
+The polarity of HCl facilitates its dissolution in water because the polar water molecules can form hydrogen bonds with the partial negative charge on the chlorine atom of HCl, thereby increasing its solubility.
What are the implications of HCl’s polarity on its chemical behavior?
+The polarity of HCl influences its reactivity, particularly in its ability to donate a proton (H+), making it a strong acid. It also affects its physical properties, such as its boiling point, due to stronger intermolecular forces between polar molecules.