Phosphate Valence Electrons
Phosphate, which is a polyatomic ion composed of phosphorus and oxygen, has a complex electronic structure that plays a crucial role in its chemical properties and behavior. Understanding the valence electrons of phosphate is essential for grasping its reactivity, stability, and interactions with other molecules.
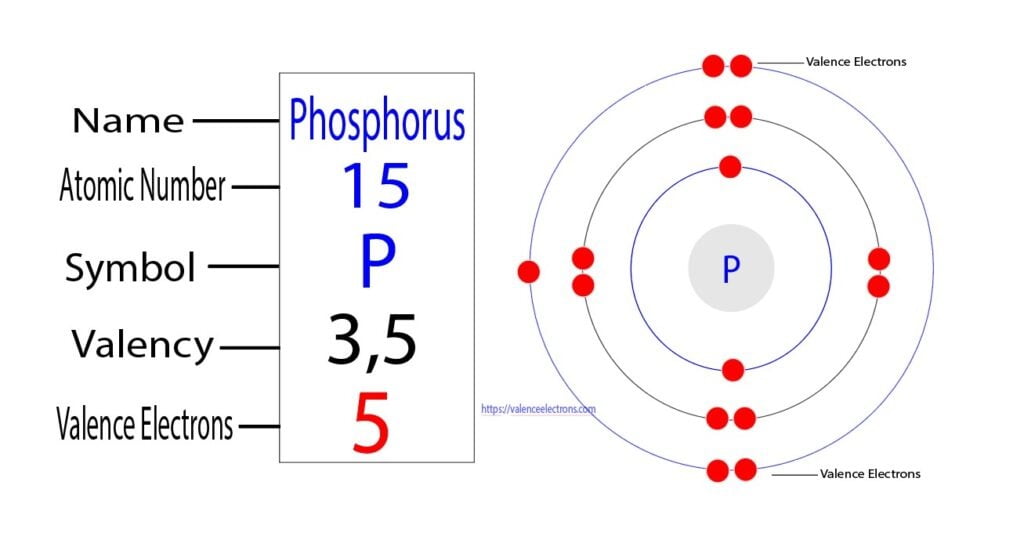
To begin with, let’s examine the electronic configuration of phosphorus, the central atom in the phosphate ion. Phosphorus is in group 15 of the periodic table and has an atomic number of 15. Its electronic configuration can be represented as 1s² 2s² 2p⁶ 3s² 3p³. The outermost energy level, or valence shell, of phosphorus contains five electrons: two in the 3s orbital and three in the 3p orbitals.
When phosphorus forms a phosphate ion (PO₄³⁻), it undergoes significant changes in its electronic configuration. The phosphate ion is formed when a phosphorus atom bonds with four oxygen atoms, resulting in a tetrahedral arrangement. In this process, phosphorus shares its valence electrons with oxygen atoms to form covalent bonds.
Each oxygen atom in the phosphate ion has a high electronegativity, which means it has a strong tendency to attract electrons towards itself. As a result, the oxygen atoms pull the shared electrons closer, effectively gaining a partial negative charge. This leaves the phosphorus atom with a partial positive charge.
The phosphate ion has a -3 charge, indicating that it has gained three additional electrons. These extra electrons are distributed among the oxygen atoms, with each oxygen atom carrying a partial negative charge. The phosphorus atom, on the other hand, has lost three electrons, resulting in a partial positive charge.
To determine the number of valence electrons in the phosphate ion, we need to consider the electronic configuration of the phosphorus atom and the oxygen atoms. Phosphorus has five valence electrons, while each oxygen atom has six valence electrons. Since there are four oxygen atoms in the phosphate ion, the total number of valence electrons contributed by oxygen is 4 x 6 = 24.
However, the phosphate ion has a -3 charge, which means it has gained three additional electrons. These extra electrons are added to the total valence electrons, resulting in a total of 5 (from phosphorus) + 24 (from oxygen) + 3 (additional electrons) = 32 valence electrons.
The valence electrons in the phosphate ion are distributed among the phosphorus and oxygen atoms. The phosphorus atom has a formal charge of +5, while each oxygen atom has a formal charge of -1 (except for one oxygen, which has a formal charge of -2 in some resonance structures). The distribution of valence electrons in the phosphate ion can be represented as follows:
- Phosphorus: 5 valence electrons (shared with oxygen)
- Oxygen (x4): 24 valence electrons (shared with phosphorus and among themselves)
It’s essential to note that the phosphate ion has multiple resonance structures, which means that the valence electrons are delocalized among the oxygen atoms. This delocalization of electrons contributes to the stability of the phosphate ion and its ability to form strong bonds with other molecules.
In conclusion, the phosphate ion has a complex electronic structure, with 32 valence electrons distributed among the phosphorus and oxygen atoms. Understanding the valence electrons of phosphate is crucial for grasping its chemical properties, reactivity, and interactions with other molecules. The unique distribution of valence electrons in the phosphate ion makes it an essential component in various biological and chemical processes, including nucleic acid synthesis, energy transfer, and mineral formation.
Valence Electron Distribution in Phosphate
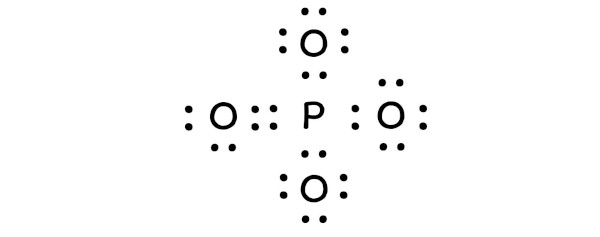
The valence electron distribution in the phosphate ion can be represented using Lewis structures. The Lewis structure of phosphate shows the arrangement of valence electrons around the phosphorus and oxygen atoms. In this structure, the phosphorus atom is bonded to four oxygen atoms, with each bond representing a shared pair of electrons.
The Lewis structure of phosphate can be drawn as follows:
- Phosphorus (P) is the central atom, bonded to four oxygen atoms (O)
- Each oxygen atom is bonded to the phosphorus atom through a single bond, representing a shared pair of electrons
- The oxygen atoms also have lone pairs of electrons, which are not involved in bonding
The total number of valence electrons in the phosphate ion can be calculated by adding the valence electrons of the phosphorus atom and the oxygen atoms. As mentioned earlier, the phosphorus atom has five valence electrons, while each oxygen atom has six valence electrons.
Using the Lewis structure, we can calculate the formal charge on each atom in the phosphate ion. The formal charge is a measure of the charge on an atom in a molecule, taking into account the number of valence electrons and the number of electrons involved in bonding.
The formal charge on the phosphorus atom can be calculated as follows:
- Phosphorus has five valence electrons
- Phosphorus is bonded to four oxygen atoms, with each bond representing a shared pair of electrons
- Phosphorus has a formal charge of +5 (5 valence electrons - 4 shared pairs of electrons)
The formal charge on each oxygen atom can be calculated similarly:
- Oxygen has six valence electrons
- Oxygen is bonded to the phosphorus atom through a single bond, representing a shared pair of electrons
- Oxygen has a formal charge of -1 (6 valence electrons - 1 shared pair of electrons - 4 lone pair electrons)
Chemical Properties of Phosphate

The phosphate ion exhibits several unique chemical properties due to its valence electron distribution. Some of the key chemical properties of phosphate include:
- Basicity: Phosphate is a weak base, which means it can accept protons (H⁺) to form a conjugate acid. The basicity of phosphate is due to the lone pairs of electrons on the oxygen atoms, which can donate electrons to form a bond with a proton.
- Acidity: Phosphate can also act as a weak acid, which means it can donate protons (H⁺) to form a conjugate base. The acidity of phosphate is due to the partial positive charge on the phosphorus atom, which can attract electrons from a base.
- Stability: Phosphate is a relatively stable ion, due to the delocalization of valence electrons among the oxygen atoms. This delocalization of electrons contributes to the stability of the phosphate ion and its ability to form strong bonds with other molecules.
The chemical properties of phosphate are essential for its biological functions, including DNA synthesis, energy transfer, and bone mineralization. The unique valence electron distribution of phosphate makes it an ideal component for these processes, allowing it to form strong bonds with other molecules and facilitate chemical reactions.
Biological Importance of Phosphate
Phosphate plays a vital role in many biological processes, including:
- DNA synthesis: Phosphate is a component of nucleic acids, such as DNA and RNA. It forms the backbone of these molecules, providing a framework for the attachment of nucleotides.
- Energy transfer: Phosphate is involved in energy transfer reactions, such as the synthesis of ATP (adenosine triphosphate) from ADP (adenosine diphosphate) and Pi (inorganic phosphate).
- Bone mineralization: Phosphate is a component of bone tissue, where it forms a hard, calcified matrix with calcium ions.
The biological importance of phosphate is due to its unique valence electron distribution, which allows it to form strong bonds with other molecules and facilitate chemical reactions. The phosphate ion is an essential component of many biological processes, and its absence or deficiency can lead to various diseases and disorders.
What is the valence electron distribution of the phosphate ion?
+The phosphate ion has 32 valence electrons, distributed among the phosphorus and oxygen atoms. The phosphorus atom has five valence electrons, while each oxygen atom has six valence electrons.
What are the chemical properties of phosphate?
+Phosphate exhibits several unique chemical properties, including basicity, acidity, and stability. Its basicity is due to the lone pairs of electrons on the oxygen atoms, while its acidity is due to the partial positive charge on the phosphorus atom.
What is the biological importance of phosphate?
+Phosphate plays a vital role in many biological processes, including DNA synthesis, energy transfer, and bone mineralization. Its unique valence electron distribution makes it an essential component of these processes, allowing it to form strong bonds with other molecules and facilitate chemical reactions.
