Lone Pairs Of Nh3
The concept of lone pairs is crucial in understanding the chemical behavior of molecules, and ammonia (NH3) is a prime example where lone pairs play a significant role. NH3, or ammonia, is a molecule composed of one nitrogen atom and three hydrogen atoms. The nitrogen atom in NH3 is bonded to three hydrogen atoms through covalent bonds, and it also has a lone pair of electrons. This lone pair is a pair of electrons that is not involved in the bonding between atoms, residing on the nitrogen atom.
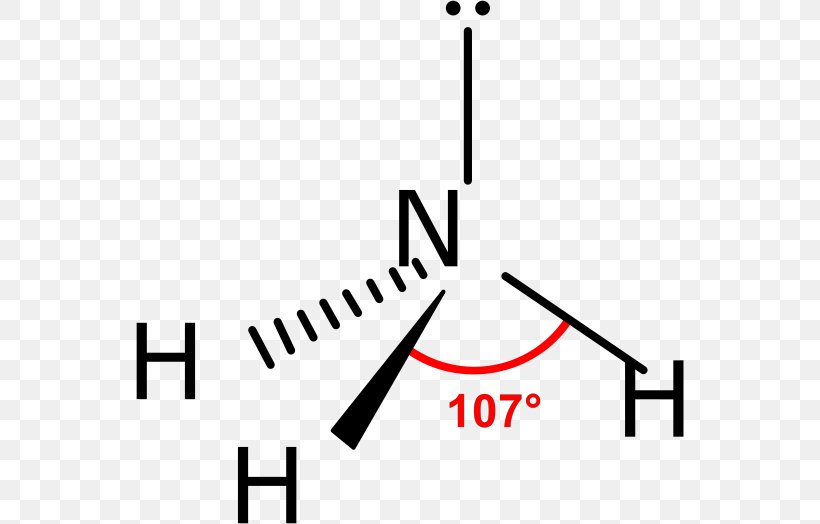
To understand the significance of the lone pair in NH3, let’s first delve into the molecular structure of ammonia. The molecule has a trigonal pyramidal shape, which is primarily due to the presence of the lone pair on the nitrogen atom. According to VSEPR (Valence Shell Electron Pair Repulsion) theory, electron pairs around a central atom arrange themselves to minimize repulsions between them. In NH3, there are three bonding pairs (from the N-H bonds) and one lone pair. These four electron pairs (three bonding pairs and one lone pair) are arranged in a tetrahedral fashion around the nitrogen atom to minimize repulsion. However, because the lone pair is not involved in a bond and does not have the same spatial requirements as bonding pairs, it occupies more space than the bonding pairs, resulting in a trigonal pyramidal shape for the molecule.
The lone pair on the nitrogen atom of NH3 is also responsible for its basicity. In chemistry, a base is defined as a substance that can accept a proton (H+ ion). The lone pair of electrons on the nitrogen atom of ammonia can accept a proton, thereby acting as a base and making NH3 a basic compound. This reaction is often represented as NH3 + H+ → NH4+, where the ammonia molecule accepts a proton to form the ammonium ion.
Moreover, the presence of the lone pair influences the physical properties of ammonia, such as its boiling point. Intermolecular forces, specifically hydrogen bonding, are stronger in substances where hydrogen is bonded to a highly electronegative atom like nitrogen, and the presence of a lone pair enhances the ability of nitrogen to participate in hydrogen bonding. Although the lone pair itself is not directly involved in hydrogen bonding, the electronegative nitrogen atom with its lone pair can create a significant dipole moment in the NH3 molecule, facilitating stronger intermolecular interactions and thus a higher boiling point compared to molecules of similar size that do not exhibit hydrogen bonding.
The reactivity of NH3 is also influenced by its lone pair. The availability of this electron pair makes nitrogen in ammonia a good nucleophile, capable of attacking electron-deficient centers in other molecules. Thisproperty is crucial in many organic reactions and biological processes.
In conclusion, the lone pair of electrons on the nitrogen atom in NH3 significantly influences the molecule’s shape, chemical properties, and reactivity. Understanding the role of lone pairs in molecules like ammonia is fundamental to appreciating their behavior in chemical reactions and theirproperties, underscoring the importance of basic principles in chemistry in explaining the complexities of molecular interactions.
Problem-Solution Framework: Managing Lone Pair Effects in Chemical Synthesis

- Problem: The presence of lone pairs can alter the reactivity and selectivity of chemical reactions.
- Solution: Understanding and predicting the effects of lone pairs allows chemists to design reactions that take advantage of these properties, enhancing the efficiency and specificity of chemical synthesis.
Expert Insight
Comparative Analysis: Lone Pairs in Different Molecules
- NH3 (Ammonia): Exhibits a lone pair that contributes to its basicity and ability to form hydrogen bonds.
- H2O (Water): Also has lone pairs on its oxygen atom, which are crucial for its high boiling point and solvent properties.
- CH4 (Methane): Lacks lone pairs on its carbon atom, resulting in a non-polar molecule with very different physical and chemical properties compared to NH3 and H2O.
Historical Evolution: Understanding Lone Pairs
- Early Chemistry: Initial understanding of molecular structure and bonding.
- Development of VSEPR Theory: Provided a framework for predicting molecular shapes based on electron pair repulsions.
- Modern Chemistry: Recognition of the critical role of lone pairs in chemical reactivity and properties.
Technical Breakdown: Molecular Orbital Theory and Lone Pairs

- Introduction to Molecular Orbitals: Describes how atomic orbitals combine to form molecular orbitals.
- Non-bonding Orbitals: Contains the lone pair electrons, which do not participate in bonding but influence molecular properties.
- Implications for Reactivity: Lone pairs in non-bonding orbitals can be involved in chemical reactions, acting as nucleophiles or participating in electron transfer reactions.
Decision Framework: Considerations for Lone Pair Effects in Chemical Design
- Identify Presence of Lone Pairs: Determine if a molecule contains lone pairs and their potential impact on reactivity.
- Predict Molecular Shape: Use VSEPR theory to estimate the molecular geometry and how lone pairs influence it.
- Assess Potential for Hydrogen Bonding: Evaluate if the molecule can participate in hydrogen bonding, affecting its physical properties.
- Consider Basicity: If applicable, assess the molecule’s potential to act as a base, accepting protons.
FAQ Section
What is the role of the lone pair in NH3's basicity?
+The lone pair on the nitrogen atom of NH3 allows it to accept a proton (H+), thereby acting as a base. This ability is fundamental to the chemical properties of ammonia.
How does the lone pair influence the physical properties of NH3?
+The lone pair contributes to the molecule's ability to form hydrogen bonds, which are stronger intermolecular forces. This results in a higher boiling point for NH3 compared to molecules that cannot form hydrogen bonds.
Can lone pairs participate in chemical reactions?
+Yes, lone pairs can participate in chemical reactions. They can act as nucleophiles, attacking electron-deficient centers in other molecules, which is a crucial aspect of many organic reactions and biological processes.
Future Trends Projection: Applications of Lone Pair Chemistry
- Advanced Materials: Understanding and manipulating lone pairs could lead to the development of new materials with unique properties.
- Pharmaceuticals: The design of drugs that exploit the lone pair properties of molecules could enhance their efficacy and selectivity.
- Energy Applications: Lone pairs might play a role in the development of more efficient energy storage and conversion technologies.
Resource Guide
For those interested in delving deeper into the world of lone pairs and their applications, the following resources are recommended: - Textbooks on Organic Chemistry: Provide a comprehensive introduction to the principles of molecular structure and reactivity. - Peer-Reviewed Journals: Offer the latest research findings and applications of lone pair chemistry. - Online Educational Platforms: Can provide interactive tutorials and visual aids to help understand complex concepts related to lone pairs and molecular structure.

