Lewis Structure For Carbon Monoxide
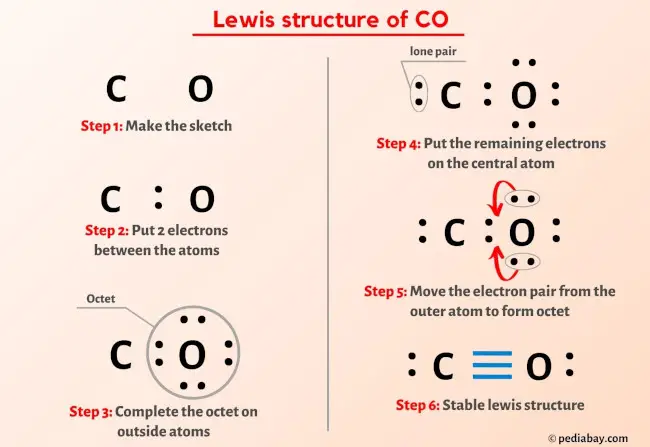
Understanding the Lewis structure for carbon monoxide (CO) is fundamental in chemistry, as it helps in visualizing the distribution of electrons within the molecule. Carbon monoxide is a diatomic molecule, consisting of one carbon atom and one oxygen atom. To draw the Lewis structure, we follow a series of steps that help us determine the arrangement of electrons.
Step 1: Determine the Total Number of Valence Electrons
First, we need to calculate the total number of valence electrons in the molecule. Carbon is in Group 14 of the periodic table and has 4 valence electrons, while oxygen is in Group 16 and has 6 valence electrons. Therefore, the total number of valence electrons in CO is 4 (from carbon) + 6 (from oxygen) = 10 valence electrons.
Step 2: Draw the Skeleton of the Molecule
The next step is to draw the skeleton of the molecule, which involves placing the atoms relative to each other. In the case of CO, the carbon and oxygen atoms are directly bonded to each other, so we draw them connected by a single bond.
Step 3: Add Electrons to the OuterShell
Now, we add the valence electrons to the outer shell of each atom, starting with the atom that is more electronegative (in this case, oxygen). We distribute the electrons such that each atom achieves a noble gas configuration, if possible, and then form bonds between atoms to achieve this stable configuration.
Step 4: Form Bonds
To form a bond between carbon and oxygen, we share a pair of electrons. This leaves us with 8 electrons (10 total electrons minus the 2 electrons used in the bond). We then distribute the remaining electrons around the atoms such that each atom has a full outer shell, which for carbon means having 8 electrons (like neon) and for oxygen means having 8 electrons as well.
Step 5: Finalize the Lewis Structure
After distributing the electrons, we should end up with a structure where carbon has 4 bonds (one to oxygen and three additional bonds that can be thought of as being to itself to fulfill the octet rule in a way that reflects the molecule’s real electron distribution) and oxygen has 2 bonds to carbon and 2 lone pairs, fulfilling the octet rule for both atoms.
However, a more accurate representation considering the real bonding in CO involves a triple bond between carbon and oxygen. This is because CO has a bond order of 3, reflecting its stability and the short bond length between the carbon and oxygen atoms. The triple bond consists of one sigma (σ) bond and two pi (π) bonds. This structure better represents the molecule’s stability and reactivity.
Corrected Lewis Structure for CO:
- The carbon atom shares 6 electrons with the oxygen atom (triple bond: 1 sigma and 2 pi bonds).
- Oxygen has 2 lone pairs of electrons, which are not involved in bonding.
- Carbon has no lone pairs in this model but is involved in the triple bond with oxygen.
This representation provides a clearer understanding of the electron distribution and bonding in the carbon monoxide molecule, aligning with its known chemical properties and reactivity.
Understanding the Bond Order:
The bond order in a molecule is defined as half the difference between the number of electrons in bonding molecular orbitals and the number of electrons in antibonding molecular orbitals. For CO, the bond order is 3, indicating a strong bond between the carbon and oxygen atoms, which is consistent with the observed physical and chemical properties of CO.
Implications of the Lewis Structure:
The Lewis structure of CO provides insights into its chemical behavior, such as its toxicity, its role as a ligand in coordination compounds, and its reactivity in various chemical reactions. Understanding the electron distribution and the type of bonding in CO is crucial for predicting its chemical properties and behavior in different environments.
In conclusion, the Lewis structure for carbon monoxide, when accurately represented with a triple bond between the carbon and oxygen atoms, offers valuable insights into the molecule’s stability, reactivity, and chemical properties. This understanding is essential for applications ranging from industrial processes to biological systems where CO plays a significant role.
When drawing Lewis structures, it's essential to consider the molecule's overall electronic structure and stability, rather than just following a set of rules. This approach helps in understanding the chemical behavior of molecules like CO and predicting their reactivity in various situations.
FAQ Section:
What is the significance of the triple bond in the CO molecule?
+The triple bond in CO signifies a strong and stable bond between the carbon and oxygen atoms, contributing to the molecule's low reactivity towards many substances and its high bond energy.
How does the Lewis structure of CO relate to its chemical properties?
+The Lewis structure indicates the distribution of electrons, which influences CO's reactivity, polarity, and ability to form complexes with metals. The structure suggests CO is a good ligand due to its ability to donate electrons through the carbon atom.
What are the implications of CO's bond order for its physical properties?
+The high bond order of 3 in CO results in a short and strong bond between carbon and oxygen, leading to high bond energy and contributing to CO's stability and low reactivity. This also affects its physical properties, such as boiling and melting points.
By examining the Lewis structure of CO in depth, we gain a comprehensive understanding of its chemical and physical properties, which are crucial for various applications in chemistry and beyond.

