How To Write Calcium Bromide Lewis Dot Structure? Step Guide
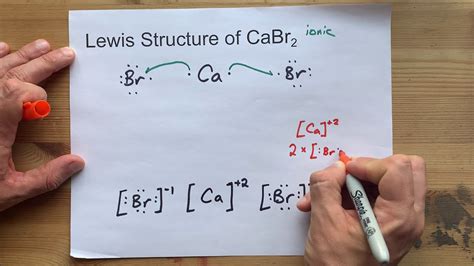
Calcium bromide, denoted by the chemical formula CaBr2, is a compound consisting of one calcium (Ca) atom and two bromine (Br) atoms. Writing the Lewis dot structure for calcium bromide involves several steps that help visualize the distribution of electrons within the molecule. This guide will walk you through the process of drawing the Lewis structure for CaBr2.
Step 1: Determine the Total Number of Valence Electrons
To start, you need to calculate the total number of valence electrons in the calcium bromide molecule. Calcium is in Group 2 of the periodic table and has 2 valence electrons. Bromine is in Group 17 and has 7 valence electrons. Since there are two bromine atoms, you multiply the number of valence electrons in one bromine atom by 2.
- Calcium (Ca): 2 valence electrons
- Bromine (Br): 7 valence electrons (each), and there are 2 bromine atoms, so 7 * 2 = 14 valence electrons
Total valence electrons = 2 (from Ca) + 14 (from 2 Br) = 16 valence electrons
Step 2: Draw the Skeleton Structure
Next, draw the basic structure of the molecule, which involves placing the atoms relative to each other. In calcium bromide, the calcium atom is typically central because it can form two bonds, one with each bromine atom, to achieve a stable electron configuration.
The skeleton structure would look something like this: Ca - Br Br
However, since we’re considering the Lewis structure, which focuses on electron distribution, we can simplify the representation to Ca with two Br atoms attached, without explicitly drawing the bonds at this stage.
Step 3: Connect the Atoms with Single Bonds
To start distributing the electrons, connect the calcium atom to each bromine atom with a single bond. Each single bond represents 2 shared electrons.
Ca - Br Br
After drawing these single bonds, 4 electrons have been used (2 for each bond), leaving 12 electrons to be distributed.
Step 4: Distribute Remaining Electrons
The remaining electrons should be distributed around the atoms to satisfy the octet rule, where each atom (except for hydrogen, which seeks 2 electrons) aims to have 8 electrons in its valence shell.
- Calcium started with 2 valence electrons and has 2 single bonds, each contributing 2 electrons, thus completing its valence shell with 8 electrons (2 original + 6 from bonds).
- Each bromine atom started with 7 valence electrons and is involved in one single bond, contributing 2 electrons from the bond. To achieve an octet, each bromine needs 6 more electrons, which are fulfilled by the remaining electrons.
Distribute the remaining 12 electrons as lone pairs around the bromine atoms. Each bromine atom will have 3 lone pairs (6 electrons) in addition to the 2 electrons from the single bond with calcium, thus achieving an octet configuration for each bromine.
The final Lewis dot structure for calcium bromide would show calcium with no lone pairs (its valence shell is filled by the single bonds to bromine), and each bromine atom surrounded by 3 lone pairs, in addition to the single bond to calcium.
Step 5: Verify Octet Rule Satisfaction
- Calcium (Ca): 2 original electrons + 6 electrons from 2 single bonds = 8 electrons, satisfying the octet rule.
- Bromine (Br): 7 original electrons + 2 electrons from the single bond = 9 electrons. However, in Lewis structures, it’s common to represent the bonding pair and then the remaining electrons as lone pairs, ensuring each atom has an octet. Thus, each bromine effectively has an octet when considering its bond and lone pairs.
By following these steps, you’ve successfully drawn the Lewis dot structure for calcium bromide (CaBr2), demonstrating how electrons are distributed within the molecule to fulfill the octet rule for each atom involved.
Example of Completed Lewis Structure:
To visually represent the Lewis structure as described:
Ca (with no lone pairs, and lines representing bonds to the bromines) - Br::: (with 3 lone pairs and a single bond to calcium) - Br::: (with 3 lone pairs and a single bond to calcium)
This structure illustrates the calcium atom bonded to two bromine atoms, with each bromine having three lone pairs of electrons, visually representing the distribution of electrons in calcium bromide.
Conclusion
Writing the Lewis dot structure for calcium bromide involves understanding the basic principles of Lewis structures, such as the importance of the octet rule, how to distribute electrons to satisfy this rule, and visually representing bonds and lone pairs. This guide has walked you through the step-by-step process of creating the Lewis structure for CaBr2, a crucial tool for understanding the molecular structure and properties of compounds.