How Does So4 2 Form Conjugate Acid? Simple Guide
To understand how SO4^2- forms a conjugate acid, we first need to grasp some basic principles of chemistry, particularly acid-base theory. The sulfate ion, SO4^2-, is a polyatomic anion that can act as a base in chemical reactions. It’s the conjugate base of the bisulfate ion (HSO4^-), which in turn is the conjugate base of sulfuric acid (H2SO4), a strong acid.
Understanding Acid-Base Chemistry
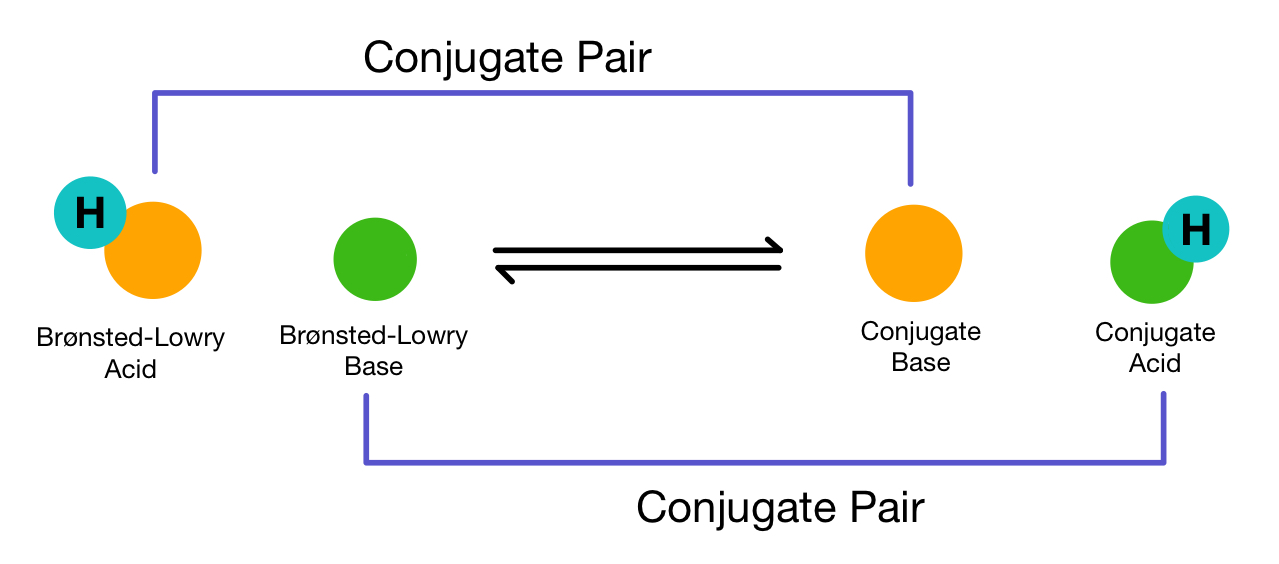
In acid-base chemistry, particularly when discussing the Bronsted-Lowry theory, an acid is defined as a species that donates a proton (H+), while a base is a species that accepts a proton. When a base accepts a proton, it forms its conjugate acid. The relationship between an acid and its conjugate base (or a base and its conjugate acid) is fundamental to understanding many chemical reactions.
The Sulfate Ion as a Base
The sulfate ion, SO4^2-, has a -2 charge, meaning it has two extra electrons compared to a neutral sulfur atom. This makes it a relatively strong base in aqueous solutions, capable of accepting protons (H+) to form a more stable species.
Formation of the Conjugate Acid
The conjugate acid of SO4^2- is formed when it accepts one proton (H+). The reaction can be represented as follows:
SO4^2- + H+ → HSO4^- (bisulfate ion)
In this reaction, SO4^2- acts as a base, accepting a proton to form the bisulfate ion (HSO4^-), which is its conjugate acid. If the bisulfate ion were to accept another proton, it would form sulfuric acid (H2SO4), a process that can be represented as:
HSO4^- + H+ → H2SO4
However, the focus here is on the formation of the conjugate acid of sulfate, which is the bisulfate ion.
Importance of Conjugate Acids in Chemistry
Conjugate acids and their corresponding bases play crucial roles in many chemical and biochemical processes. Understanding these relationships is essential for predicting and manipulating chemical reactions, whether in industrial applications, environmental science, or biological systems.
Example Applications
- Water Treatment: Understanding how sulfate forms its conjugate acid is important in water treatment processes. Sulfate and its conjugate acid, bisulfate, can influence water acidity and the efficiency of treatment chemicals.
- Biological Systems: In living organisms, sulfate is involved in various metabolic pathways. Its ability to accept and donate protons is crucial in maintaining the acid-base balance within cells and tissues.
- Chemical Industry: Sulfuric acid, the conjugate acid of bisulfate, is a key chemical in the production of numerous products, including fertilizers, detergents, and pharmaceuticals. The efficient production of sulfuric acid from sulfate requires an understanding of its acid-base chemistry.
Conclusion
In summary, the sulfate ion forms its conjugate acid, the bisulfate ion, by accepting a proton in an aqueous solution. This process is fundamental to acid-base chemistry and has implications across various fields, from environmental science and biology to the chemical industry. Understanding these principles not only enhances our appreciation of chemical reactions but also provides insights into how we can manipulate and utilize these reactions for practical applications.
What is the conjugate acid of the sulfate ion?
+The conjugate acid of the sulfate ion (SO4^2-) is the bisulfate ion (HSO4^-), which is formed when sulfate accepts a proton (H+).
Why is understanding conjugate acids important in chemistry?
+Understanding conjugate acids and their corresponding bases is crucial for predicting and manipulating chemical reactions, as well as for understanding various biological and environmental processes.
This understanding of acid-base relationships is a cornerstone of chemistry and has wide-ranging implications for both theoretical knowledge and practical applications. By grasping how ions like sulfate interact with protons, we can better understand and manage chemical reactions in multiple contexts.