H2o Electron Pair: Understand Molecular Shape
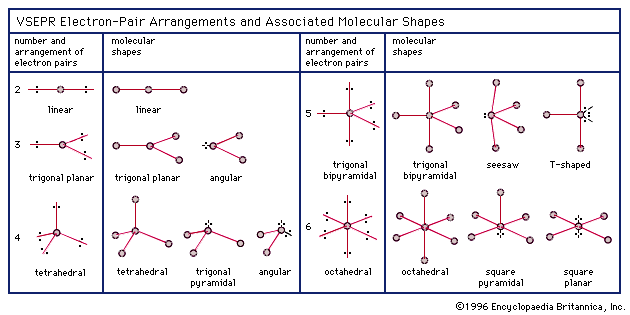
The molecular shape of a molecule is determined by the arrangement of its electron pairs. In the case of H2O, or water, the molecule has a unique shape due to the presence of two hydrogen atoms bonded to a single oxygen atom. To understand the molecular shape of H2O, it’s essential to examine the electron pair geometry and the concept of hybridization.
Electron Pair Geometry
The electron pair geometry of a molecule is the arrangement of its electron pairs in space. In H2O, the oxygen atom has six valence electrons, and each hydrogen atom has one valence electron. When the oxygen atom shares its electrons with the two hydrogen atoms, it forms two covalent bonds. The remaining four electrons on the oxygen atom form two lone pairs. These lone pairs occupy space around the oxygen atom, influencing the overall molecular shape.
The electron pair geometry of H2O can be described as tetrahedral, with the two hydrogen atoms and the two lone pairs occupying the four corners of a tetrahedron. However, since the two lone pairs are not equivalent to the two bonding pairs, the molecular shape is not a perfect tetrahedron.
Hybridization
To explain the molecular shape of H2O, it’s necessary to consider the concept of hybridization. Hybridization occurs when atomic orbitals mix to form new hybrid orbitals. In the case of oxygen, the atomic orbitals are 2s and 2p. When these orbitals mix, they form four sp3 hybrid orbitals, which are oriented in a tetrahedral arrangement.
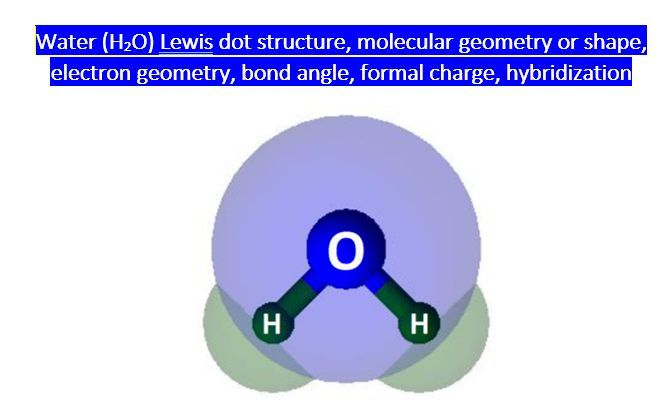
The two sp3 hybrid orbitals that participate in bonding with the hydrogen atoms are directional, meaning they point towards the hydrogen atoms. The remaining two sp3 hybrid orbitals contain the lone pairs and are oriented away from the hydrogen atoms. This results in a bent or V-shape molecular geometry, with the two hydrogen atoms at an angle of approximately 104.5 degrees.
Molecular Shape
The molecular shape of H2O is bent or V-shaped, with the two hydrogen atoms at an angle of approximately 104.5 degrees. This shape is a result of the tetrahedral electron pair geometry and the sp3 hybridization of the oxygen atom. The bent shape of H2O allows it to form hydrogen bonds with other water molecules, which is essential for its chemical and physical properties.
Key Takeaways
- The molecular shape of H2O is determined by the arrangement of its electron pairs.
- The electron pair geometry of H2O is tetrahedral, with two hydrogen atoms and two lone pairs.
- Hybridization occurs when atomic orbitals mix to form new hybrid orbitals.
- The sp3 hybridization of the oxygen atom results in a bent or V-shape molecular geometry.
- The molecular shape of H2O is essential for its chemical and physical properties, including its ability to form hydrogen bonds.
Comparison with Other Molecules
The molecular shape of H2O can be compared to other molecules with similar electron pair geometries. For example, the molecule NH3 (ammonia) has a similar tetrahedral electron pair geometry, but with three hydrogen atoms and one lone pair. The molecular shape of NH3 is also bent, but with a slightly different angle due to the presence of only one lone pair.
| Molecule | Electron Pair Geometry | Molecular Shape |
|---|---|---|
| H2O | Tetrahedral | Bent (V-shaped) |
| NH3 | Tetrahedral | Bent (V-shaped) |
| CH4 | Tetrahedral | Tetrahedral |
Historical Context
The concept of molecular shape and electron pair geometry was developed by Gilbert Newton Lewis and Irving Langmuir in the early 20th century. They introduced the idea of electron pairs and the octet rule, which states that atoms tend to gain or lose electrons to form a stable octet of electrons in their outer shell.
Over time, the concept of molecular shape has evolved to include the idea of hybridization and the use of molecular orbital theory to predict molecular geometry. Today, the study of molecular shape is an essential part of chemistry and is used to predict the properties and behavior of molecules in a wide range of fields, from materials science to pharmacology.
What is the molecular shape of H2O?
+The molecular shape of H2O is bent or V-shaped, with the two hydrogen atoms at an angle of approximately 104.5 degrees.
What is the electron pair geometry of H2O?
+The electron pair geometry of H2O is tetrahedral, with two hydrogen atoms and two lone pairs.
What is hybridization, and how does it relate to molecular shape?
+Hybridization occurs when atomic orbitals mix to form new hybrid orbitals. In the case of H2O, the sp3 hybridization of the oxygen atom results in a bent or V-shape molecular geometry.
Conclusion
In conclusion, the molecular shape of H2O is a complex phenomenon that arises from the arrangement of its electron pairs and the hybridization of the oxygen atom. Understanding the molecular shape of H2O is essential for predicting its chemical and physical properties, including its ability to form hydrogen bonds and its high boiling point. By examining the electron pair geometry and hybridization of H2O, we can gain a deeper appreciation for the intricate mechanisms that govern the behavior of molecules and the importance of molecular shape in determining their properties.