H2co Lewis Dot: Structured Diagram Guide
The H2CO Lewis dot structure is a fundamental concept in chemistry, representing the molecular structure of formaldehyde. To understand this structure, it’s essential to break down the components and follow a step-by-step approach to drawing the Lewis dot diagram.
Introduction to Formaldehyde (H2CO)
Formaldehyde, with the chemical formula H2CO, is the simplest aldehyde and one of the most common organic compounds. It’s a colorless, strong-smelling gas at room temperature and is highly reactive. Formaldehyde is used in the production of resins, plastics, and other chemicals, and it has various applications in construction, automotive, and consumer goods industries.
Basics of Lewis Dot Structures
Lewis dot structures, or electron dot diagrams, are a simple way to represent the valence electrons in an atom. They are used to show how electrons are arranged in a molecule and to predict the chemical properties of the molecule. The basic steps to draw a Lewis dot structure include:
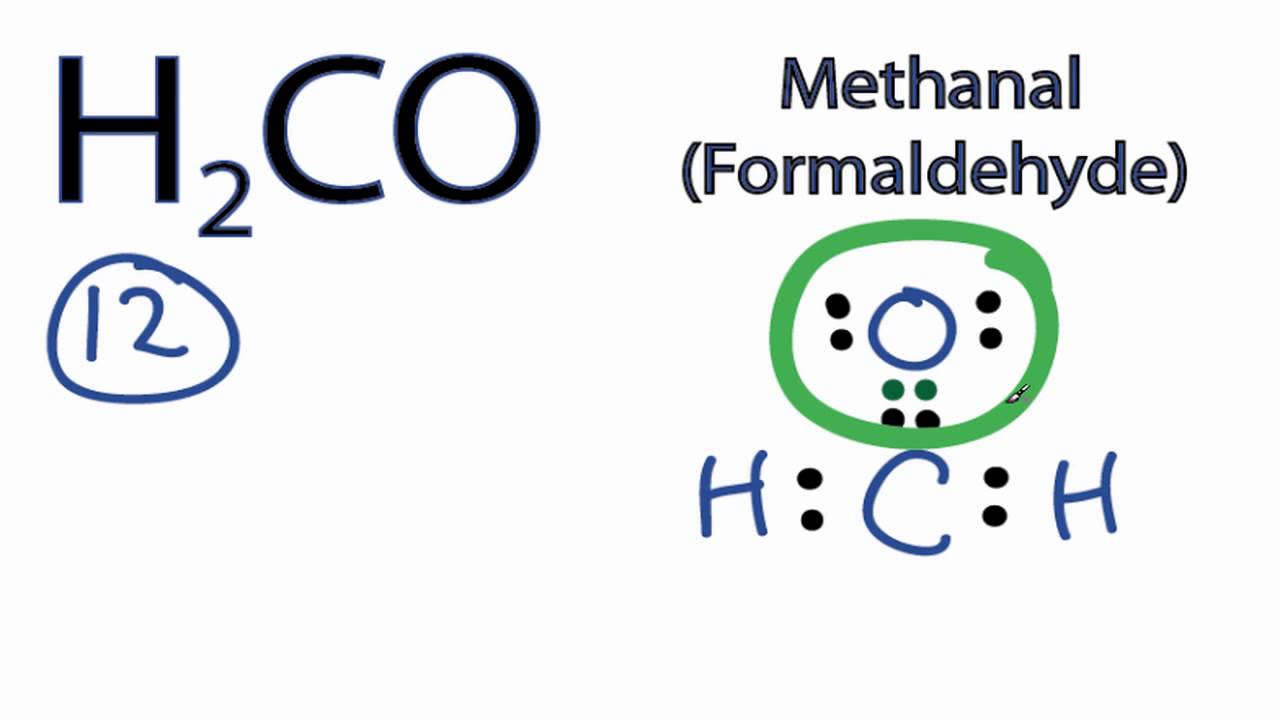
Determine the Total Number of Valence Electrons: Calculate the total number of valence electrons by adding the valence electrons of each atom in the molecule. For H2CO, hydrogen (H) has 1 valence electron, carbon © has 4, and oxygen (O) has 6. Thus, the total is 1*2 (for two hydrogens) + 4 (for carbon) + 6 (for oxygen) = 2 + 4 + 6 = 12 valence electrons.
Draw the Skeleton of the Molecule: Connect the atoms with single bonds, which represent two shared electrons. For H2CO, the carbon is the central atom, so we draw two single bonds from carbon to each hydrogen and one single bond from carbon to oxygen. This uses 4 electrons (2 for each single bond), leaving us with 12 - 4 = 8 electrons.
Add Electrons to the Atoms: Place the remaining electrons around the atoms to fill their valence shells. Hydrogen needs 2 electrons to fill its shell, carbon needs 8, and oxygen needs 8. Since we’ve already used 4 electrons for the single bonds, we distribute the remaining 8 electrons. Each hydrogen gets 2 electrons (to complete its duet), and the remaining 4 electrons are placed around oxygen because carbon will share electrons in the next steps to achieve a full octet.
Satisfy the Octet Rule for Each Atom: The octet rule states that an atom tends to have 8 electrons in its outer shell. For carbon, which is currently short of an octet, we can form a double bond with oxygen. This double bond represents 4 shared electrons, which are added to the structure without changing the number of electrons around the other atoms. Now, carbon has 8 electrons (4 from the two single bonds with hydrogen and 4 from the double bond with oxygen), and oxygen also has 8 electrons (6 non-bonding electrons and 2 from the double bond with carbon), fulfilling the octet rule for both.
Drawing the H2CO Lewis Dot Structure
Here’s how the Lewis dot structure for H2CO is drawn based on the steps above:
Carbon ©: Central atom, bonded to two hydrogens and one oxygen. It shares 4 electrons in single bonds with hydrogens and 4 electrons in a double bond with oxygen.
Hydrogen (H): Two hydrogens are each bonded to carbon with a single bond, and each has 2 non-bonding electrons (dotted pairs) to fulfill their duet.
Oxygen (O): Bonded to carbon with a double bond, and has 4 non-bonding electrons (dotted pairs) to fulfill its octet.
This results in a structure where carbon and oxygen share a double bond, and each hydrogen shares a single bond with carbon. The structure looks like this:
H - C = O
• • • • • •
Where each line represents a bond (single or double), and the dots represent non-bonding (lone pair) electrons.
Conclusion
Understanding the Lewis dot structure of H2CO is essential for recognizing its chemical properties and reactivity. By following the step-by-step guide provided, one can accurately draw the structure, ensuring that each atom adheres to the octet rule (except for hydrogen, which follows the duet rule). The ability to draw and interpret Lewis dot structures is a fundamental skill in chemistry, pivotal for understanding molecular interactions and predicting the outcomes of chemical reactions.
FAQ Section
What are the main steps to draw a Lewis dot structure for H2CO?
+The main steps include determining the total number of valence electrons, drawing the skeleton of the molecule, adding electrons to the atoms to fill their valence shells, and satisfying the octet rule for each atom.
Why is the double bond between carbon and oxygen necessary in the H2CO Lewis dot structure?
+The double bond is necessary to fulfill the octet rule for both carbon and oxygen. It ensures that each atom has 8 electrons in its outer shell, thereby achieving stability.
What is the significance of understanding the Lewis dot structure of H2CO?
+Understanding the Lewis dot structure of H2CO is crucial for recognizing its chemical properties and reactivity. It helps in predicting the outcomes of chemical reactions involving formaldehyde and is essential for understanding its industrial and biological applications.
In conclusion, mastering the art of drawing Lewis dot structures like that of H2CO not only enhances one’s understanding of molecular chemistry but also lays a solid foundation for exploring more complex compounds and reactions.


