Energy Unit Joule
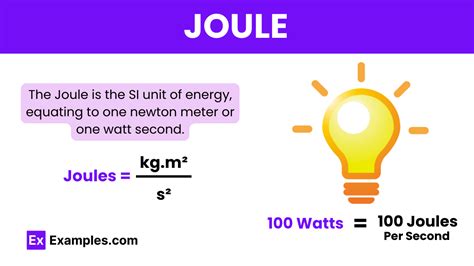
The joule, denoted by the symbol J, is the SI unit of energy, work, and heat. It is a fundamental concept in physics and engineering, and its understanding is crucial for a wide range of applications, from the smallest scales of atomic and subatomic particles to the vast scales of cosmic phenomena. In this comprehensive overview, we will delve into the definition, history, and applications of the joule, as well as its relationships with other physical quantities.
Definition and History
The joule is defined as the energy expended (or work done) when a force of one newton is applied over a distance of one meter. This definition is rooted in the concept of work, which is a measure of the energy transferred from one object to another through a force applied over a distance. The joule was named after James Prescott Joule, an English physicist who made significant contributions to the study of energy and its relationship to heat in the 19th century. Joule’s work laid the foundation for the law of conservation of energy, which states that energy cannot be created or destroyed, only transformed from one form to another.
Applications and Uses
The joule has numerous applications across various fields of science and engineering. It is used to measure the energy content of foods, the energy expenditure of the human body, the energy efficiency of appliances and vehicles, and the energy output of power plants, among many other examples. In electrical engineering, the joule is used to quantify the energy stored in capacitors and the energy dissipated as heat in resistors. In thermal engineering, it is used to measure the energy transferred as heat, which is crucial for designing heating and cooling systems.
Relationship with Other Physical Quantities
The joule is closely related to other physical quantities such as watt (the unit of power), which is defined as one joule per second (J/s). This relationship highlights the distinction between energy (the capacity to do work) and power (the rate at which work is done). The joule is also related to the calorie, a unit of energy commonly used in nutrition and chemistry, where 1 calorie = 4.184 joules. Understanding these relationships is essential for converting between different units of energy and for solving problems involving energy transformations.
Measurement and Conversion
Measuring energy in joules can be done through various methods, depending on the form of energy being considered. For mechanical energy, it can involve measuring force and distance. For thermal energy, it might involve measuring temperature changes and specific heat capacities. Converting between joules and other units of energy (such as calories, kilowatt-hours, or British Thermal Units) is straightforward with the use of conversion factors.
Joule in Different Forms of Energy
- Kinetic Energy: The energy an object possesses due to its motion. It can be calculated using the formula 0.5 * m * v^2, where m is the mass of the object and v is its velocity.
- Potential Energy: The energy an object has due to its position or state. For example, gravitational potential energy can be calculated using the formula m * g * h, where m is the mass, g is the acceleration due to gravity, and h is the height above the reference point.
- Thermal Energy: The total internal kinetic and potential energy of the particles in an object due to their random motion. It is related to the temperature of the object.
- Electrical Energy: The energy caused by the movement of electrons. It can be calculated in terms of voltage, current, and time.
Conclusion
In conclusion, the joule is a fundamental unit of energy that underpins our understanding of the physical world. Its applications are diverse and critical, from the design of more efficient energy systems to the study of cosmic phenomena. Understanding the joule and its relationships with other physical quantities is essential for advancing in fields such as physics, engineering, and environmental science. By grasping the concept of energy and its various forms, we can better appreciate the intricate balance of our universe and work towards sustainable energy solutions for the future.
FAQ Section

What is the joule, and how is it defined?
+The joule is the SI unit of energy, work, and heat. It is defined as the energy expended when a force of one newton is applied over a distance of one meter.
How is the joule related to other units of energy?
+The joule is related to other units of energy through conversion factors. For example, 1 calorie equals 4.184 joules, and 1 watt-hour (Wh) equals 3600 joules.
What are some common applications of the joule in science and engineering?
+The joule has numerous applications, including measuring the energy content of foods, the energy efficiency of appliances, and the energy output of power plants. It is also used in electrical engineering to quantify energy stored in capacitors and dissipated as heat in resistors.
How is energy measured in joules?
+Measuring energy in joules can be done through various methods, including measuring force and distance for mechanical energy, temperature changes and specific heat capacities for thermal energy, and voltage, current, and time for electrical energy.
In the realm of physics and engineering, understanding the joule and its applications is not just about grasping a unit of measurement but about comprehending the fundamental nature of energy and its transformations. As we continue to explore and innovate, the joule remains a foundational concept, guiding our understanding of the world and our place within it.

