Co32 Resonance Structure
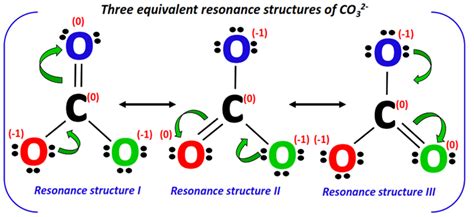
The CO32- ion, also known as the carbonate ion, is a fundamental component in various chemical and biological processes. To understand its properties and behavior, it’s essential to explore its resonance structures. Resonance structures are a set of Lewis structures that contribute to the overall electronic structure of a molecule or ion. These structures are used to describe the delocalization of electrons in molecules, which is crucial for understanding their stability, reactivity, and other properties.
Introduction to Resonance
Resonance in chemistry refers to the representation of a molecule by multiple Lewis structures that differ only in the arrangement of their electrons, without changing the positions of the atoms. This concept is vital because it helps in understanding that the actual structure of a molecule is not just one of these representations but a hybrid of all possible resonance structures. The carbonate ion (CO32-) is a classic example where resonance plays a significant role.
CO32- Resonance Structures
The carbonate ion has three resonance structures, each contributing to its overall stability. These structures are:
- Structure 1: In this structure, one of the oxygen atoms is double-bonded to the carbon atom, while the other two oxygen atoms are single-bonded to the carbon and each have a negative charge.
O=
/
O- - C - O-
- Structure 2: Here, the double bond is between the carbon and a different oxygen atom compared to Structure 1, with the same distribution of single bonds and negative charges.
O- - C = O
/
O-
- Structure 3: This structure has the double bond between the carbon and the third oxygen atom, maintaining the same pattern of single bonds and negative charges as the previous structures.
O-
/
O = C - O-
Importance of Resonance in CO32-
The resonance structures of the carbonate ion are crucial for understanding its properties:
- Stability: The delocalization of the negative charge across all three oxygen atoms increases the stability of the ion. This is because the charge is not localized on a single atom, reducing the energy of the system.
- Reactivity: The resonance structures influence the reactivity of the carbonate ion. For instance, its ability to act as a nucleophile in chemical reactions can be understood through its resonance forms.
- Symmetry: The actual structure of the CO32- ion is a hybrid of the resonance structures, resulting in a trigonal planar geometry with equal C-O bond lengths. This symmetry is a direct consequence of the delocalization of electrons.
Applications and Biological Significance
The carbonate ion plays a significant role in various biological and chemical processes:
- Biological Processes: In the human body, the carbonate ion is a vital component of the buffering system that helps maintain blood pH. It is also involved in the formation of shells in marine organisms.
- Chemical Processes: CO32- is used in the manufacture of glass, as a component in cement, and in the production of sodium carbonate (soda ash), which has numerous industrial applications.
Conclusion
In conclusion, the resonance structures of the CO32- ion are fundamental to understanding its chemical properties and biological significance. The delocalization of electrons, as represented by the different resonance structures, contributes to the ion’s stability and reactivity, making it a crucial component in various chemical and biological processes. This understanding underscores the importance of resonance in chemistry and its application in explaining the behavior of molecules and ions.
FAQ Section
What is the significance of resonance structures in chemistry?
+Resonance structures are significant in chemistry because they help in understanding the delocalization of electrons in molecules, which is crucial for explaining their stability, reactivity, and other properties.
How does the resonance of the CO32- ion contribute to its stability?
+The resonance of the CO32- ion increases its stability by delocalizing the negative charge across all three oxygen atoms. This delocalization reduces the energy of the system, making the ion more stable.
What are some biological applications of the carbonate ion?
+The carbonate ion is involved in the buffering system of the human body, helping to maintain blood pH. It also plays a role in the formation of shells in marine organisms.
Further Reading
For those interested in delving deeper into the realm of resonance structures and their applications, there are numerous resources available that explore the theoretical foundations, experimental evidence, and practical implications of this concept in chemistry. Understanding resonance is key to grasping many of the fundamental principles of chemistry and biology, offering a rich area of study for both students and researchers alike.


