Co32 Bond Angle
The CO32- ion, also known as the carbonate ion, is a fascinating molecule that plays a crucial role in various chemical and biological processes. One of the most interesting aspects of this ion is its bond angle, which is a fundamental property that determines its shape and reactivity.
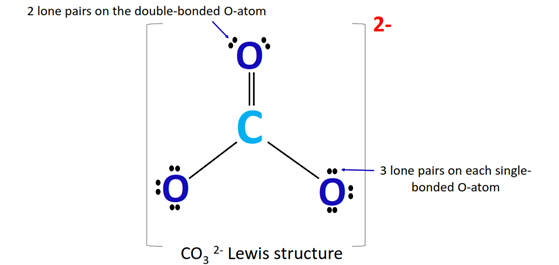
To understand the bond angle of CO32-, let’s first examine its molecular structure. The carbonate ion consists of one central carbon atom bonded to three oxygen atoms, with a total of 24 valence electrons. The carbon atom is sp2 hybridized, meaning that it has three equivalent hybrid orbitals that are oriented in a trigonal planar geometry. Each oxygen atom is bonded to the central carbon atom through a sigma (σ) bond, and there is also a delocalized pi (π) bond that is distributed evenly among the three oxygen atoms.
The bond angle of CO32- is typically around 120 degrees, which is a characteristic of a trigonal planar molecule. This means that the three oxygen atoms are arranged in a plane around the central carbon atom, with each oxygen atom separated from its neighbors by an angle of 120 degrees. This bond angle is a result of the sp2 hybridization of the carbon atom and the delocalization of the pi bond among the three oxygen atoms.
But why is the bond angle of CO32- so important? The answer lies in its reactivity and biological significance. The carbonate ion is a key player in many chemical reactions, including the formation of shells and skeletons in marine organisms. The bond angle of CO32- influences its ability to form hydrogen bonds and interact with other molecules, which is crucial for its biological function.
In addition to its biological significance, the bond angle of CO32- also has important implications for its chemical reactivity. The trigonal planar geometry of the carbonate ion makes it a relatively stable molecule, but it can still undergo various chemical reactions, such as protonation and complexation. The bond angle of CO32- affects its ability to participate in these reactions, making it an important factor in determining its chemical properties.
To gain a deeper understanding of the bond angle of CO32-, let’s examine some of the key factors that influence its value. One of the most important factors is the hybridization of the carbon atom, which determines the orientation of the hybrid orbitals and the resulting bond angle. The delocalization of the pi bond among the three oxygen atoms also plays a crucial role in determining the bond angle, as it affects the distribution of electrons among the oxygen atoms.
Another important factor that influences the bond angle of CO32- is the presence of electron-withdrawing or electron-donating groups. These groups can alter the distribution of electrons among the oxygen atoms, leading to changes in the bond angle. For example, the presence of an electron-withdrawing group, such as a hydrogen bond acceptor, can decrease the bond angle of CO32-, while the presence of an electron-donating group, such as a hydrogen bond donor, can increase the bond angle.
In conclusion, the bond angle of CO32- is a fascinating property that plays a crucial role in determining its shape, reactivity, and biological significance. The trigonal planar geometry of the carbonate ion, with a bond angle of around 120 degrees, is a result of the sp2 hybridization of the carbon atom and the delocalization of the pi bond among the three oxygen atoms. Understanding the factors that influence the bond angle of CO32- is essential for appreciating its chemical and biological properties, and for predicting its behavior in various chemical reactions.
The bond angle of CO32- is a critical property that affects its reactivity and biological function. Understanding the factors that influence this property is essential for appreciating the chemical and biological properties of the carbonate ion.
What is the bond angle of CO32-?
+The bond angle of CO32- is typically around 120 degrees, which is a characteristic of a trigonal planar molecule.
Why is the bond angle of CO32- important?
+The bond angle of CO32- influences its ability to form hydrogen bonds and interact with other molecules, which is crucial for its biological function and chemical reactivity.
What factors influence the bond angle of CO32-?
+The hybridization of the carbon atom, the delocalization of the pi bond among the three oxygen atoms, and the presence of electron-withdrawing or electron-donating groups are some of the key factors that influence the bond angle of CO32-.
In the following sections, we will delve deeper into the chemical and biological properties of CO32-, and explore its significance in various fields, including chemistry, biology, and environmental science.
Chemical Properties of CO32-

The chemical properties of CO32- are determined by its molecular structure and bond angle. The trigonal planar geometry of the carbonate ion makes it a relatively stable molecule, but it can still undergo various chemical reactions, such as protonation and complexation. The bond angle of CO32- affects its ability to participate in these reactions, making it an important factor in determining its chemical properties.
Step 1: Protonation of CO32-
The protonation of CO32- occurs when a hydrogen ion (H+) binds to one of the oxygen atoms, forming a bicarbonate ion (HCO3-).
Step 2: Complexation of CO32-
The complexation of CO32- occurs when it binds to a metal ion, such as calcium (Ca2+) or magnesium (Mg2+), forming a complex ion.
Biological Significance of CO32-
The biological significance of CO32- is closely related to its chemical properties. The carbonate ion plays a crucial role in many biological processes, including the formation of shells and skeletons in marine organisms. The bond angle of CO32- influences its ability to form hydrogen bonds and interact with other molecules, which is crucial for its biological function.
Advantages of CO32- in Biological Systems
- Forms hydrogen bonds with other molecules, which is crucial for its biological function
- Interacts with other ions, such as calcium and magnesium, to form complex ions
- Plays a crucial role in the formation of shells and skeletons in marine organisms
Disadvantages of CO32- in Biological Systems
- Can be affected by changes in pH, which can alter its chemical properties
- Can be competed with by other ions, such as phosphate, for binding sites
- Can be limited by the availability of calcium and magnesium ions
In conclusion, the bond angle of CO32- is a critical property that affects its reactivity and biological function. Understanding the factors that influence this property is essential for appreciating the chemical and biological properties of the carbonate ion. The trigonal planar geometry of CO32- makes it a relatively stable molecule, but it can still undergo various chemical reactions, such as protonation and complexation. The bond angle of CO32- influences its ability to form hydrogen bonds and interact with other molecules, which is crucial for its biological function and chemical reactivity.
The bond angle of CO32- is a critical property that affects its reactivity and biological function, and understanding the factors that influence this property is essential for appreciating the chemical and biological properties of the carbonate ion.