Ch4 Vsepr Model
The VSEPR model, which stands for Valence Shell Electron Pair Repulsion, is a fundamental concept in chemistry that helps predict the shape of molecules. This model is based on the idea that electron pairs in the valence shell of an atom repel each other and arrange themselves to minimize repulsion. In this section, we will delve into the details of the VSEPR model, exploring its principles, applications, and limitations.
Introduction to VSEPR
The VSEPR model was developed by Ronald Gillespie and Ronald Nyholm in the 1950s. It is a simple, yet powerful tool for predicting the shapes of molecules based on the number of electron pairs around a central atom. The model assumes that electron pairs, whether they are bonding pairs or lone pairs, occupy specific positions in space to minimize repulsion between them.
Basic Principles of VSEPR
- Electron Pairs Repel Each Other: The fundamental principle of VSEPR is that electron pairs repel each other due to their negative charge. This repulsion is what drives the arrangement of electron pairs in space.
- Electron Pairs Occupy Specific Positions: According to VSEPR, electron pairs arrange themselves in specific positions around a central atom to maximize the distance between them and minimize repulsion.
- Lone Pairs and Bonding Pairs are Equivalent: In the VSEPR model, lone pairs (non-bonding electron pairs) and bonding pairs (electrons involved in covalent bonds) are considered equivalent in terms of their ability to repel other electron pairs.
- The Shape of a Molecule is Determined by the Arrangement of Electron Pairs: The overall shape of a molecule is determined by the arrangement of its electron pairs around the central atom(s), which in turn determines the positions of the atoms in space.
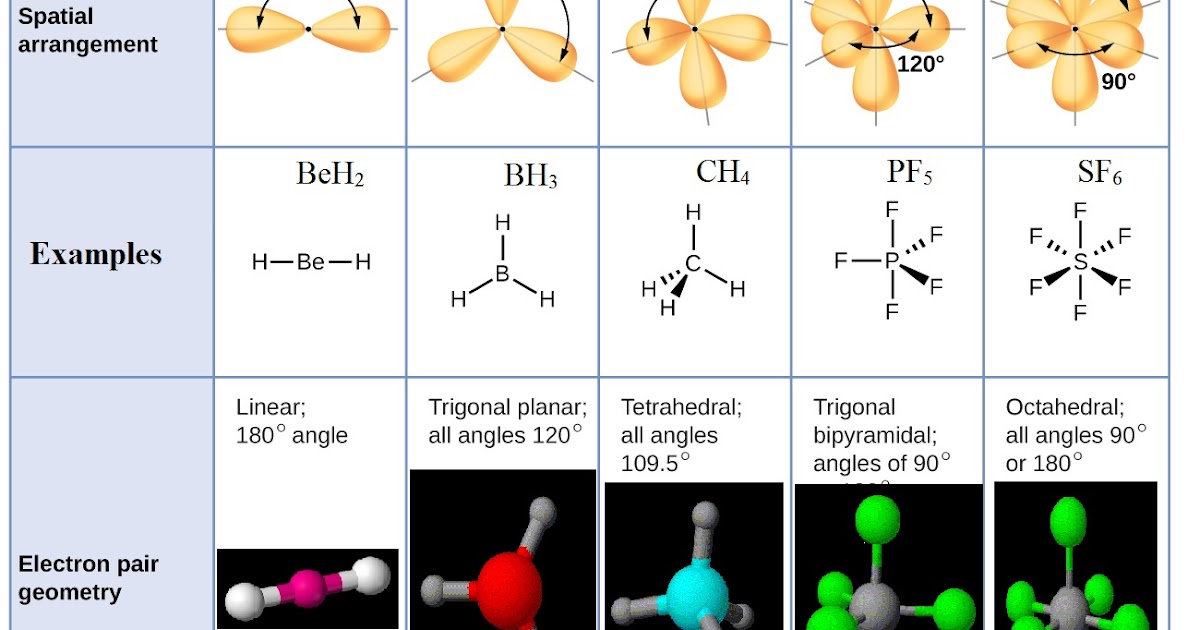
VSEPR Geometries
The VSEPR model predicts several common molecular geometries based on the number of electron pairs around a central atom. These include:
- Linear: Two electron pairs, resulting in a linear shape (e.g., CO2).
- Trigonal Planar: Three electron pairs, leading to a trigonal planar shape (e.g., BF3).
- Tetrahedral: Four electron pairs, resulting in a tetrahedral shape (e.g., CH4).
- Trigonal Bipyramidal: Five electron pairs, leading to a trigonal bipyramidal arrangement (e.g., PF5).
- Octahedral: Six electron pairs, resulting in an octahedral shape (e.g., SF6).
Applications of VSEPR
The VSEPR model has numerous applications in chemistry, including:
- Predicting Molecular Shapes: The most direct application of VSEPR is predicting the shapes of molecules, which is crucial for understanding their physical and chemical properties.
- Understanding Chemical Reactivity: The shape of a molecule can influence its reactivity. For example, the accessibility of a functional group can depend on the molecular shape.
- Interpreting Spectroscopic Data: Molecular shape can affect the interpretation of spectroscopic data, such as IR and NMR spectra.
Limitations of VSEPR
While the VSEPR model is a powerful tool for predicting molecular shapes, it has its limitations:
- Simplification: VSEPR simplifies the complexities of electron distribution and interaction, which can lead to inaccuracies in some cases.
- Does Not Account for Other Factors: The model primarily focuses on electron pair repulsions and does not consider other factors that can influence molecular shape, such as orbital hybridization and the effects of multiple bonds.
Real-World Examples and Case Studies
- Bent vs. Linear Shape of Water: The VSEPR model explains why water (H2O) has a bent shape due to the two lone pairs on the oxygen atom, which repel the bonding pairs and result in an angle of approximately 104.5 degrees between the hydrogen atoms.
- Tetrahedral Shape of Methane: Methane (CH4) has a tetrahedral shape because the four bonding pairs of electrons around the carbon atom are arranged to maximize distance and minimize repulsion, resulting in equal bond angles of 109.5 degrees.
Historical Evolution of VSEPR
The development of the VSEPR model marked a significant advancement in the understanding of molecular geometry. Over the years, the model has been refined and expanded upon, incorporating insights from quantum mechanics and molecular orbital theory. The evolution of computational chemistry has also enabled more precise predictions of molecular shapes and properties, complementing the VSEPR model.
Future Trends and Developments
As computational power increases and theoretical models become more sophisticated, the prediction of molecular shapes and properties is becoming increasingly accurate. Future developments are likely to integrate the VSEPR model with advanced computational methods, providing a more detailed understanding of molecular geometry and its role in chemical reactivity and physical properties.
Step-by-Step Guide to Applying VSEPR
- Determine the Central Atom: Identify the atom in the molecule that is bonded to the most other atoms.
- Count Electron Pairs: Count the number of electron pairs (both bonding and lone pairs) around the central atom.
- Apply VSEPR Rules: Use the VSEPR model to predict the arrangement of these electron pairs and hence the molecular geometry.
- Consider Lone Pairs: Remember that lone pairs occupy more space than bonding pairs and can significantly affect the molecular shape.
- Draw the Molecule: Draw the molecule according to the predicted geometry, taking care to represent the relative positions of atoms in space accurately.
Key Takeaways
- The VSEPR model is a foundational concept in chemistry that helps predict molecular shapes based on electron pair repulsions.
- Understanding the VSEPR model is crucial for predicting physical and chemical properties of molecules.
- The model has applications in various fields of chemistry, from predicting reactivity to interpreting spectroscopic data.
Frequently Asked Questions
What is the main principle of the VSEPR model?
+The main principle of the VSEPR model is that electron pairs around a central atom arrange themselves to minimize repulsion, thus determining the shape of the molecule.
How does the VSEPR model account for lone pairs in predicting molecular shape?
+The VSEPR model treats lone pairs as equivalent to bonding pairs in terms of repulsion. Lone pairs are considered to occupy more space than bonding pairs, which can significantly affect the predicted shape of a molecule.
What are some limitations of the VSEPR model?
+The VSEPR model simplifies the complex interactions between electrons and does not account for other factors such as orbital hybridization and the effects of multiple bonds, which can lead to inaccuracies in predicting molecular shapes.
In conclusion, the VSEPR model is a vital tool in chemistry that provides insights into the shapes of molecules. Its principles and applications are fundamental to understanding chemical properties and reactivity. While it has limitations, the VSEPR model remains a cornerstone of chemical education and research, offering a straightforward and effective method for predicting molecular geometries. As chemistry continues to evolve, the integration of the VSEPR model with modern computational methods will further enhance our understanding of molecular structure and its role in chemical phenomena.