Carbonate Ion Guide: Complete Lewis Formula
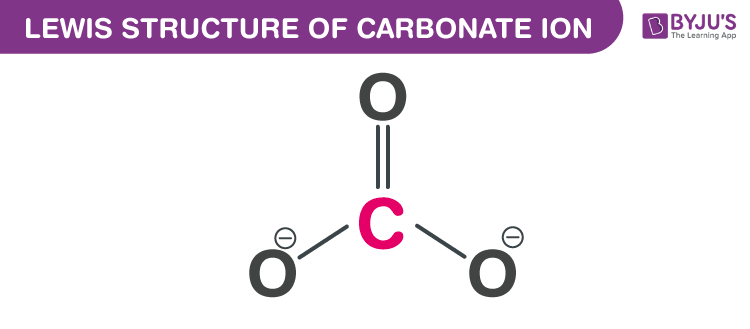
The carbonate ion, with the chemical formula CO3^2-, is a fundamental component in various chemical and biological processes. Understanding its structure is crucial for grasping its reactivity and role in different environments. The Lewis formula, also known as the Lewis structure, is a graphical representation that illustrates the bonding between atoms of a molecule and the lone pairs of electrons that may exist. In the case of the carbonate ion, its Lewis formula provides valuable insights into its electronic configuration and the nature of its chemical bonds.
Electronic Configuration of Carbon and Oxygen
To construct the Lewis formula of the carbonate ion, it’s essential to understand the electronic configurations of its constituent atoms, carbon © and oxygen (O). Carbon has an atomic number of 6, which means it has 6 electrons. Its electronic configuration is 1s^2 2s^2 2p^2. Oxygen, with an atomic number of 8, has 8 electrons, and its electronic configuration is 1s^2 2s^2 2p^4.
Constructing the Lewis Formula of CO3^2-
The carbonate ion has a total of 24 electrons: 6 from carbon and 18 from the three oxygen atoms (6 from each oxygen). Additionally, it carries a -2 charge, meaning it has 2 more electrons than the neutral carbon and oxygen atoms would contribute.
Step 1: Determine the Central Atom
- Carbon is less electronegative than oxygen and can form four bonds (using its s and three p orbitals), making it the central atom.
Step 2: Draw Single Bonds
- Three single bonds are drawn from carbon to each of the three oxygen atoms. Each single bond represents 2 shared electrons, accounting for 6 electrons.
Step 3: Add Lone Pairs
- After forming the single bonds, 18 electrons remain. Each oxygen atom, having 6 electrons from the bond and needing 8 for a full outer shell, requires 2 more electrons as a lone pair. This accounts for 6 electrons (2 lone pairs * 3 oxygen atoms).
Step 4: Distribute Remaining Electrons
- With 12 electrons now distributed, there are 6 electrons remaining (24 total electrons - 6 in bonds - 6 in lone pairs = 12, but we only accounted for the bonds and one set of lone pairs, so 12 are placed and 12 remain to fully satisfy octets and the charge). These are placed on the oxygen atoms as additional lone pairs, but to satisfy the octet rule for carbon and to minimize formal charges, a more stable structure involves double bonds.
Step 5: Form Double Bonds and Resonance Structures
- To minimize formal charges and satisfy the octet rule, one of the bonds between carbon and an oxygen atom can be made into a double bond, moving 2 of the remaining electrons into this bond. However, this would leave two of the oxygen atoms with a negative formal charge and one with no charge, which is not ideal. The carbonate ion’s actual structure involves resonance, where the double bond between carbon and one oxygen is delocalized across all three oxygen atoms. This means drawing two additional structures where the double bond is between carbon and each of the other oxygen atoms, and then combining these structures to show the average distribution of electrons.
Resonance in Carbonate Ion
The resonance structures of the carbonate ion are crucial for understanding its stability and reactivity. By delocalizing the negative charge across the three oxygen atoms, the ion achieves greater stability than if the charge were localized on a single atom. This delocalization also contributes to the planar, trigonal geometry of the ion, where the carbon atom is at the center, and the three oxygen atoms are arranged at equal distances around it.
Practical Applications and Biological Importance
The carbonate ion plays a significant role in various biological and chemical processes. It is a key component in the bicarbonate buffering system, which helps maintain the acid-base balance in blood and other bodily fluids. In the environment, carbonate ions are involved in the formation of limestone and other sedimentary rocks and play a role in the carbon cycle, influencing the Earth’s climate.
Conclusion
The Lewis formula of the carbonate ion provides a clear visual representation of its electronic structure, highlighting the distribution of electrons, the nature of its chemical bonds, and the delocalization of charge through resonance. This understanding is essential for appreciating the ion’s reactivity, its role in biological systems, and its significance in environmental and geological processes.
Frequently Asked Questions
What is the chemical formula of the carbonate ion?
+The chemical formula of the carbonate ion is CO3^2-.
Why does the carbonate ion have a -2 charge?
+The carbonate ion has a -2 charge because it has 2 more electrons than the neutral combination of carbon and oxygen atoms. These extra electrons are distributed across the oxygen atoms, contributing to the ion’s stability.
What is the significance of resonance in the carbonate ion’s structure?
+Resonance in the carbonate ion delocalizes the negative charge across the three oxygen atoms, enhancing the ion’s stability and contributing to its planar geometry. This delocalization is crucial for understanding the ion’s reactivity and biological importance.
How does the carbonate ion contribute to the bicarbonate buffering system in the human body?
+The carbonate ion, in the form of bicarbonate (HCO3^-), plays a critical role in maintaining the acid-base balance in blood and other bodily fluids. It acts as a buffer, helping to neutralize excess hydrogen ions and prevent significant changes in pH levels.

