Becl2 Molecular Geometry
The molecular geometry of BeCl2, also known as beryllium chloride, is a fundamental concept in chemistry that helps us understand the arrangement of atoms in space. To determine the molecular geometry of BeCl2, we need to consider the number of valence electrons, the hybridization of the central atom, and the repulsions between electron pairs.
BeCl2 has a total of 16 valence electrons: 4 from the beryllium atom (Be) and 6 from each of the two chlorine atoms (Cl). The Lewis structure of BeCl2 shows that the beryllium atom is bonded to two chlorine atoms through single covalent bonds, with no lone pairs on the central atom.
The central atom, beryllium, has only four valence electrons, which are involved in bonding with the two chlorine atoms. This means that the beryllium atom undergoes sp hybridization, resulting in two hybrid orbitals that are oriented at an angle of 180° to each other. Each hybrid orbital overlaps with a 3p orbital from a chlorine atom, forming a σ (sigma) bond.
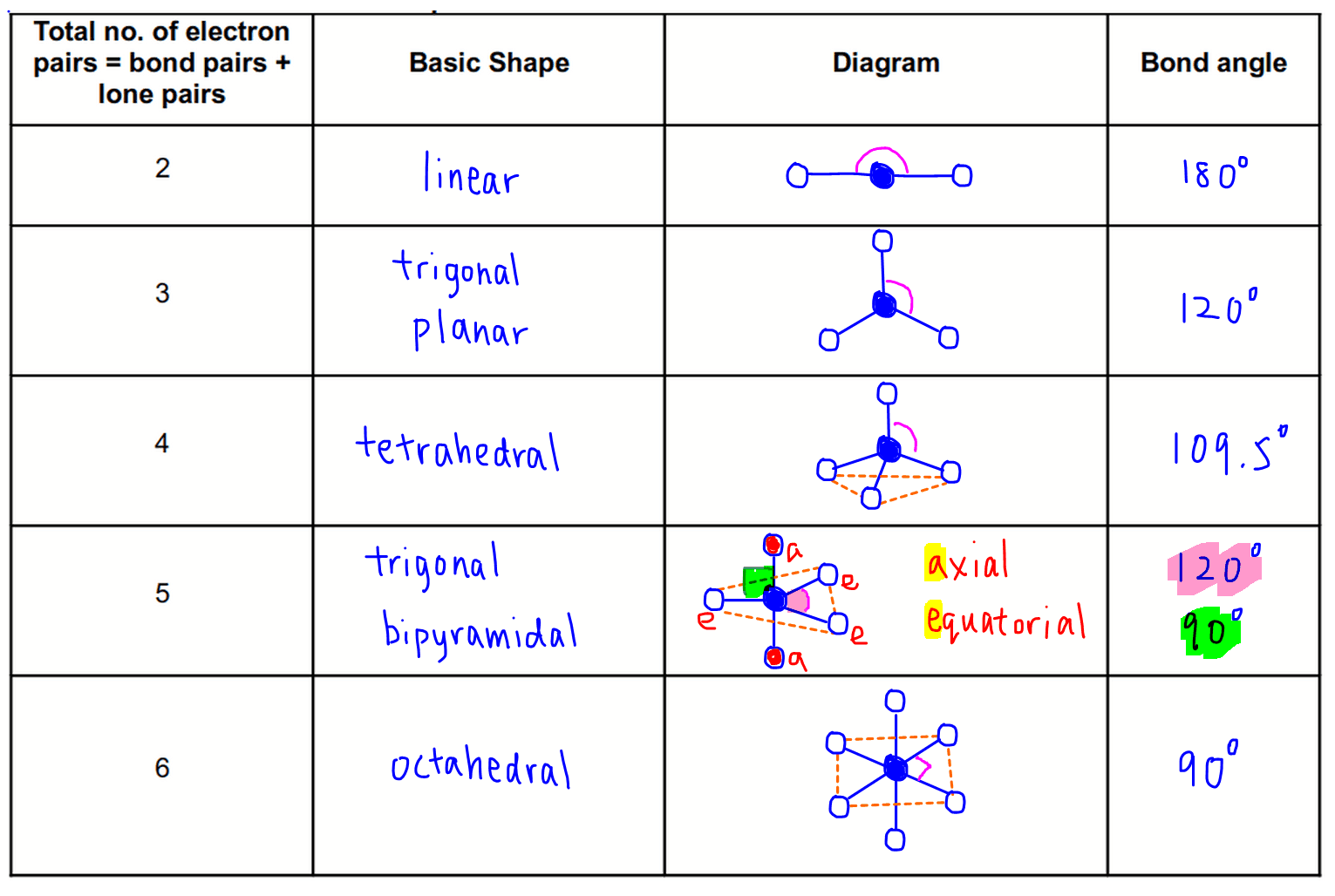
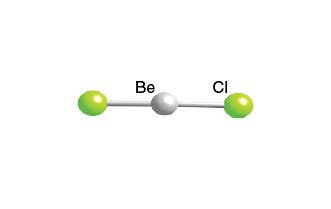
Since there are no lone pairs on the central atom, the molecular geometry of BeCl2 is determined solely by the arrangement of the two bonding pairs. According to VSEPR (Valence Shell Electron Pair Repulsion) theory, the two bonding pairs will arrange themselves to minimize repulsions, resulting in a linear molecular geometry.
In a linear molecular geometry, the two chlorine atoms are arranged on either side of the beryllium atom, with a bond angle of 180°. This arrangement allows the bonding pairs to be as far apart as possible, minimizing repulsions and resulting in a stable molecular geometry.
The linear molecular geometry of BeCl2 is also consistent with its physical properties. BeCl2 is a colorless, crystalline solid with a high melting point, indicating a high degree of symmetry and a strong bonding pattern.
Key Points: * BeCl2 has a linear molecular geometry, with a bond angle of 180°. * The central atom, beryllium, undergoes sp hybridization, resulting in two hybrid orbitals. * The molecular geometry is determined solely by the arrangement of the two bonding pairs, with no lone pairs on the central atom. * The linear molecular geometry is consistent with the physical properties of BeCl2, including its high melting point and crystalline structure.
Comparison with Other Molecules
| Molecule | Molecular Geometry | Hybridization | Bond Angle |
|---|---|---|---|
| BeCl2 | Linear | sp | 180° |
| CO2 | Linear | sp | 180° |
| H2O | Bent | sp3 | 104.5° |
The comparison with other molecules highlights the importance of considering the specific bonding patterns and hybridization schemes when determining molecular geometry.
FAQ Section
What is the molecular geometry of BeCl2?
+The molecular geometry of BeCl2 is linear, with a bond angle of 180°.
What type of hybridization does the central atom undergo?
+The central atom, beryllium, undergoes sp hybridization.
How does the molecular geometry of BeCl2 compare to that of CO2?
+Both BeCl2 and CO2 have linear molecular geometries, but the underlying bonding patterns and hybridization schemes differ.
In conclusion, the molecular geometry of BeCl2 is a fundamental concept in chemistry that helps us understand the arrangement of atoms in space. By considering the number of valence electrons, the hybridization of the central atom, and the repulsions between electron pairs, we can determine the molecular geometry of BeCl2 as linear, with a bond angle of 180°.